A kind of method for enzymatic synthesis of (s)-2-aminobutanamide
A technology for aminobutanamide and enzymatic synthesis, which is applied in the field of enzymatic synthesis of -2-aminobutanamide, can solve the problems of low yield and few synthesis reports, and achieves high ee value, high stereoselectivity, and high specificity. sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
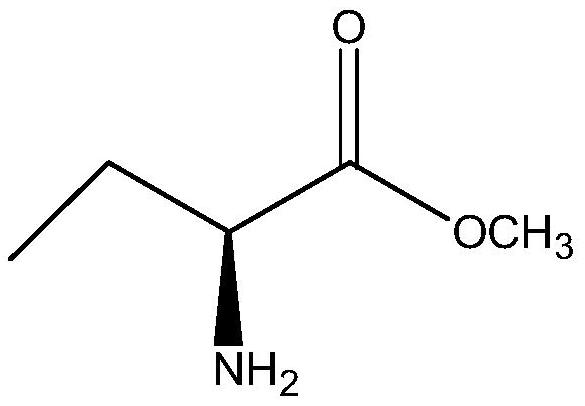
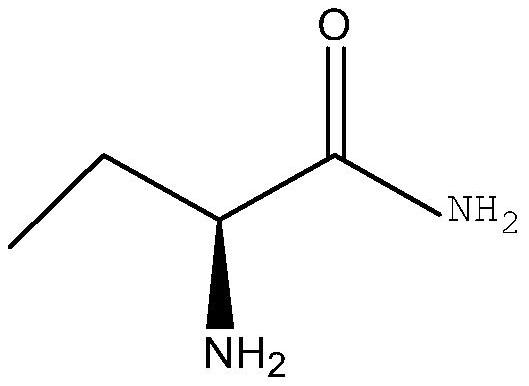
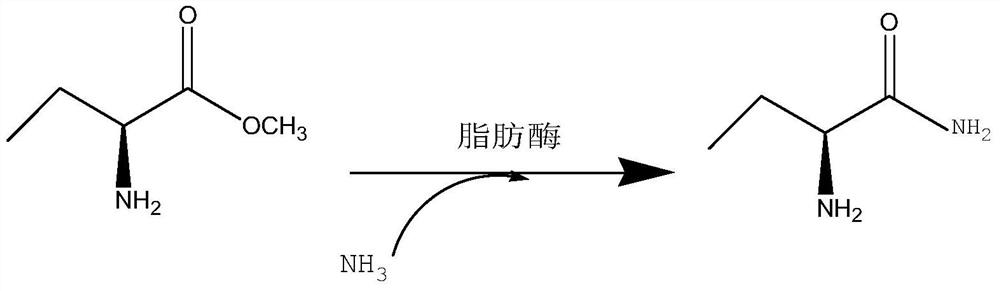
[0027] In a clean reactor, 2.1g (S)-2-aminobutyric acid methyl ester, 1.56g carbamide and 20mL isopropanol were added in sequence, and then 0.2g immobilized lipase was added, and methacrylic acid porous resin was used as Immobilize the material, then control the temperature to stir under the condition of 35 ° C, keep the water bath for 24 h, take samples for HPLC analysis, and the conversion rate is greater than 95% (chromatographic column: ODS-34.6nm×250nm, UV 200nm detection; mobile phase: phosphate buffer solution (PH=2.4), acetonitrile:methanol=80:10), after the reaction, the immobilized lipase was recovered by centrifugal filtration, and the filtrate was subjected to vacuum distillation The solvent was removed to obtain 1.62 g of the corresponding product (S)-2-aminobutanamide with a chiral purity content of 99.5%.
Embodiment 2
[0029] In a 250mL clean round-bottomed flask, 10.3g (S)-2-aminobutyric acid methyl ester, 8g ammonium carbamate and 100mL isopropanol were sequentially added to immobilize lipase 1g, and methacrylic acid porous resin was used as the immobilization material. Then control the temperature to stir at 35°C, keep the water bath for 24 hours, stop the reaction, take samples for HPLC analysis, the conversion rate is greater than 95%, centrifugal filtration to remove the immobilized lipase for recovery, and the collected filtrate is put into another clean In the reaction flask, the filtrate (reaction solution) was heated to 80°C for about 1 hour, and then 1 g of activated carbon was added for decolorization treatment for 15 minutes. After suction filtration, the collected filtrate was distilled under reduced pressure to remove the solvent, and methanol was used to distill the residue. The product was rinsed to remove impurities and dried to obtain 7.63 g of (S)-2-aminobutanamide as a wh...
Embodiment 3
[0031]In a 250mL clean round-bottomed flask, 10.3g (S)-2-aminobutyric acid methyl ester, 8g ammonium carbamate and 100mL tert-butanol were sequentially added to immobilize lipase 1g, and methacrylic acid porous resin was used as the immobilization material. The enzyme activity can reach ≥10000PLU / g, and the particle size of the immobilized lipase is 0.5mm, and then the temperature is controlled at 40°C for stirring, the reaction is kept in a water bath for 20h, the reaction is stopped, and sampling is carried out for HPLC analysis. The conversion rate is greater than 96%, centrifugal filtration to remove the immobilized lipase for recovery, the collected filtrate is put into another clean reaction bottle, the filtrate (reaction solution) is heated to 75°C for about 1h, and then 1.5g of activated carbon is added for decolorization for 15 minutes. , after suction filtration, the collected filtrate was distilled under reduced pressure to remove the solvent, and the distilled resid...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com