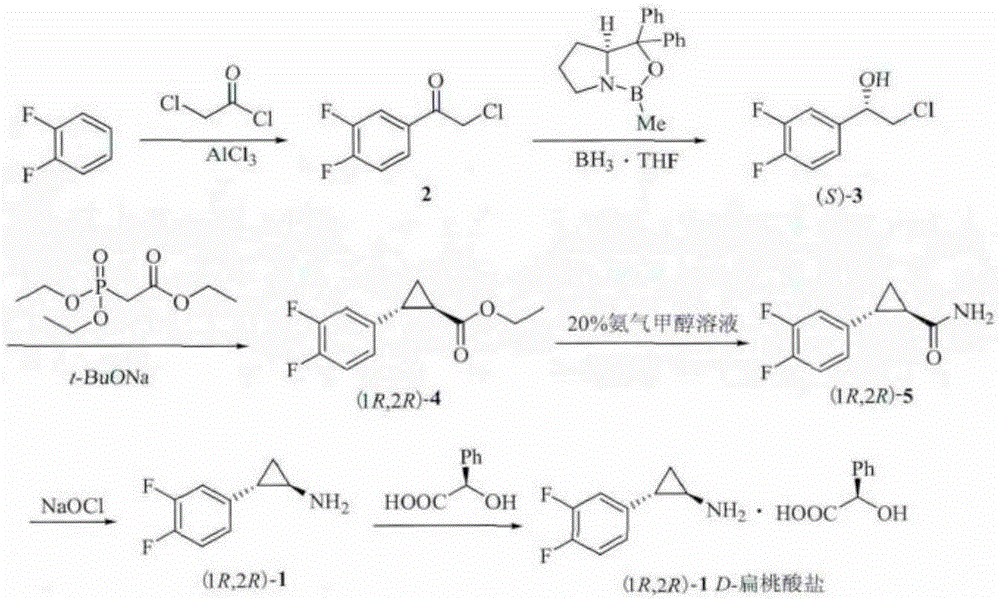
Method for preparing chiral 2-chloro-3,4-difluorophenethyl alcohol
A technology for difluorophenethyl alcohol and difluoroacetophenone is applied in the field of preparing chiral 2-chloro-3,4-difluorophenethyl alcohol, and achieves the advantages of avoiding harsh reaction conditions, high yield and good practical industrial application value. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The shake flask production technology of embodiment 1 ketoreductase (KRED) enzyme powder
[0027] The gene fragment (synthesized by Shanghai Jierui Bioengineering Co., Ltd.) containing the ketoreductase gene (SEQ ID NO.1 to 23) was ligated with the digested product of the pET28a plasmid, and transformed into competent E.coli BL21(DE3) The strains were screened to obtain positive clones, which were inoculated into 4 mL liquid LB medium containing ampicillin resistance and activated overnight (37° C., 200 rpm).
[0028] Take the overnight culture, transfer it to 100mL liquid LB medium containing ampicillin antibody at 1 / 100 inoculum size, culture at 37°C with shaking at 200rpm until OD 600 When the value reached 0.6, IPTG was added and the cultivation was continued overnight at 30°C. The cells were collected by centrifugation, and suspended in 10 mL of phosphate buffer (2 mM, pH 7.0). The cell suspension was ultrasonically disrupted in an ice bath for 10 minutes, centri...
Embodiment 2
[0029] Embodiment 2 Ketoreductase (SEQ ID NO.1~21) milligram level reaction
[0030] In a 5mL reaction flask, add 10mg of the substrate 2-chloro-3,4-difluoroacetophenone and 0.1mL of isopropanol, after the substrate is completely dissolved, add 1.2ml of TEA-HCl buffer solution (0.1M, pH7.0), 0.1mgNAD + , 0.1mgNADP + (dissolved in 0.1ml of buffer), then add 20mg of glucose and 2mg of glucose dehydrogenase, and finally, respectively add 2mg of the ketoreductase KRED enzyme powder obtained in Example 1 (0.5U / mg, dissolved in 0.1ml of buffer) , Shaking the reaction at 30°C for 20h.
[0031] Get the product after reaction and carry out HPLC analysis, the productive rate of detection product and product ee value, result is as shown in table 1:
[0032] Table 1 Yield and product ee value of different ketoreductase reactions
[0033]
[0034] Yield label description: + stands for 1%-20% yield, ++ stands for 20%-50% yield, +++ stands for 50%-80% yield, ++++ stands for 80%-95% yi...
Embodiment 3 100
[0038] Embodiment 3 100 milligram level preparation technology
[0039] In a 5ml reaction flask, add 150mg of the substrate 2-chloro-3,4-difluoroacetophenone and 0.3ml of isopropanol, after the substrate is completely dissolved, add 1.2ml of TEA-HCl buffer (0.1M, pH7.0), 7.5mg ketoreductase powder (SEQ ID NO.9) (dissolved in 0.1ml buffer), 0.75mgNAD + (dissolved in 0.1ml of buffer solution), at 30°C, a magnetic stirring reaction was performed, and two reaction times were set: 0h and 20h.
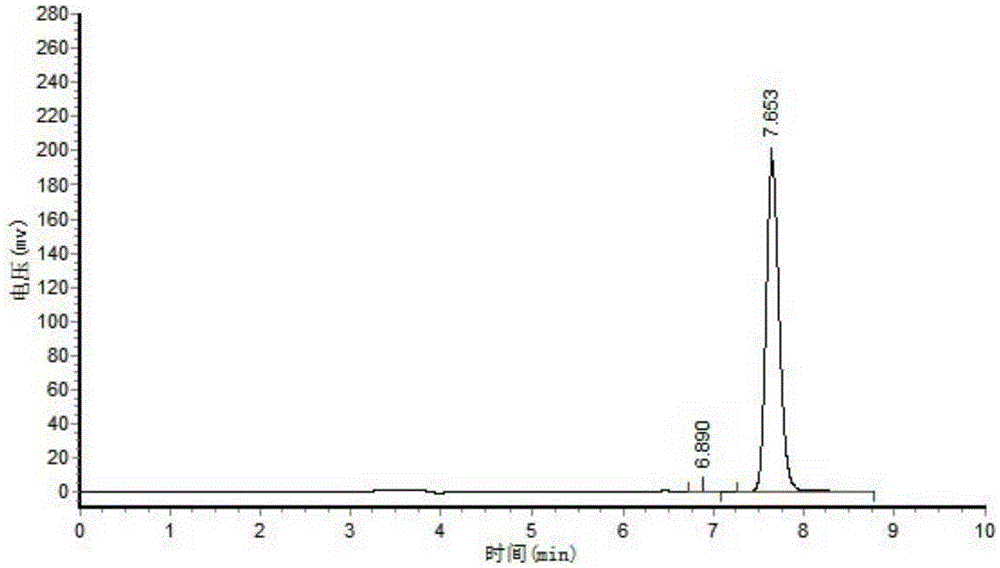
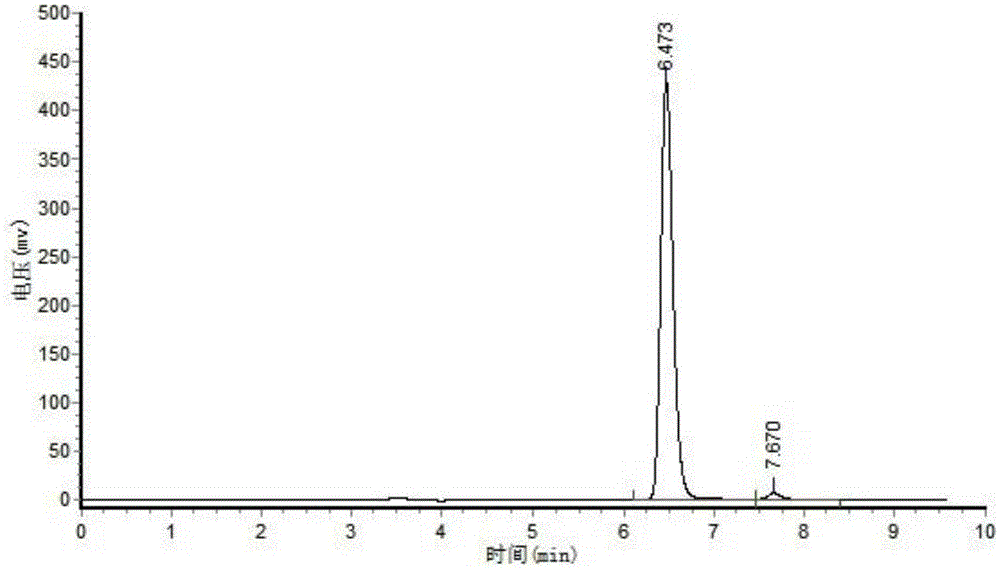
[0040] Sampling after reaction carries out HPLC analysis to product, and result is as follows: figure 1 and figure 2 shown;
[0041] figure 1For the analysis result of the product obtained after reacting for 0 hours, the substance at 7.67 minutes in the figure is the substrate;
[0042] figure 2 It is the analysis result of the product obtained after 20 hours of reaction. In the figure, the substance with a retention time of 6.47 minutes is the product, and the final conversion rate ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com