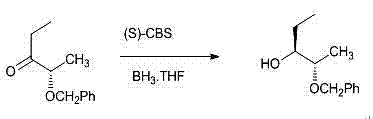
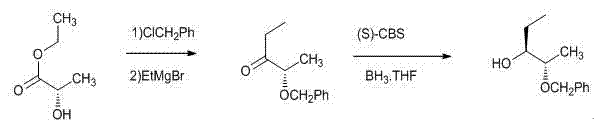
Method for preparing (2S, 3R)-2-benzyloxy-3-pentanol as intermediate of posaconazole
A technology of posaconazole and benzyloxy, which is applied in the field of preparation of the intermediate-2-benzyloxy-3-pentanol, which can solve the unfavorable production cost of posaconazole raw materials and the difficulty of chiral inducers low cost, low impurity, and low cost of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1: Preparation of (2S)-2-benzyloxy-3-pentanone
[0033]
[0034] Add 8g (0.2mol) of sodium hydroxide powder and 118ml of toluene to the reaction flask, add 1.5g of tetrabutylammonium iodide under stirring, then add 11.8g (0.1mol) of S-ethyl lactate dropwise under temperature control below 10°C, dropwise After finishing, continue to insulate and stir for 20 min. 12.6 g (0.1 mol) of benzyl chloride was added dropwise at a temperature controlled below 10°C. After the drop was completed, the reaction was stirred at a temperature of 0°C to 10°C for about 30 hours. Filtrate, distill the filtrate to remove the solvent under reduced pressure, and add the residue dropwise to a tetrahydrofuran solution of ethylmagnesium bromide (1M, 110ml) prepared in advance and cooled to below -10°C to keep the temperature at -10°C to -5°C, about After 0.5h dropwise addition, continue to maintain the temperature for 6 hours, stop the reaction, extract the reaction with saturated am...
Embodiment 2
[0035] Example 2: Preparation of (2S)-2-benzyloxy-3-pentanone
[0036]
[0037]Add 8g (0.2mol) of sodium hydroxide powder and 118ml of toluene to the reaction flask, add 1.5g of tetrabutylammonium bromide under stirring, then add 11.8g (0.1mol) of S-ethyl lactate dropwise under temperature control below 10°C, dropwise After finishing, continue to insulate and stir for 20 min. 11.3 g (0.09 mol) of benzyl chloride was added dropwise at a temperature controlled below 10°C. After the drop was complete, the reaction was stirred at a temperature of 0°C to 10°C for about 30 hours. Filtrate, distill the filtrate to remove the solvent under reduced pressure, and add the residue dropwise to a tetrahydrofuran solution of ethylmagnesium bromide (1M, 110ml) prepared in advance and cooled to below -10°C to keep the temperature at -10°C to -5°C, about After 0.5 h of dropwise addition, continue to maintain the temperature for 10 hours, stop the reaction, extract the reaction with satura...
Embodiment 3
[0038] Example 3: Preparation of (2S)-2-benzyloxy-3-pentanone
[0039]
[0040] Add 3.6 g (0.15 mol) of sodium hydride, 50 ml of tetrahydrofuran, and 25 ml of DMF into the reaction flask, add 11.8 g (0.1 mol) of S-ethyl lactate dropwise under stirring in an ice bath, and continue to insulate and stir for 30 min. 12.6 g (0.1 mol) of benzyl chloride was added dropwise at a temperature controlled below 10°C. After the drop was complete, the reaction was stirred at a temperature of 0°C to 10°C for about 20 hours. Filtrate, distill the filtrate to remove the solvent under reduced pressure, and add the residue dropwise to a tetrahydrofuran solution of ethylmagnesium bromide (1M, 110ml) prepared in advance and cooled to below -10°C to keep the temperature at -10°C to -5°C, about After 0.5h dropwise addition, continue to maintain the temperature for 6 hours, stop the reaction, extract the reaction with saturated ammonium chloride, extract with ethyl acetate, wash the organic phas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com