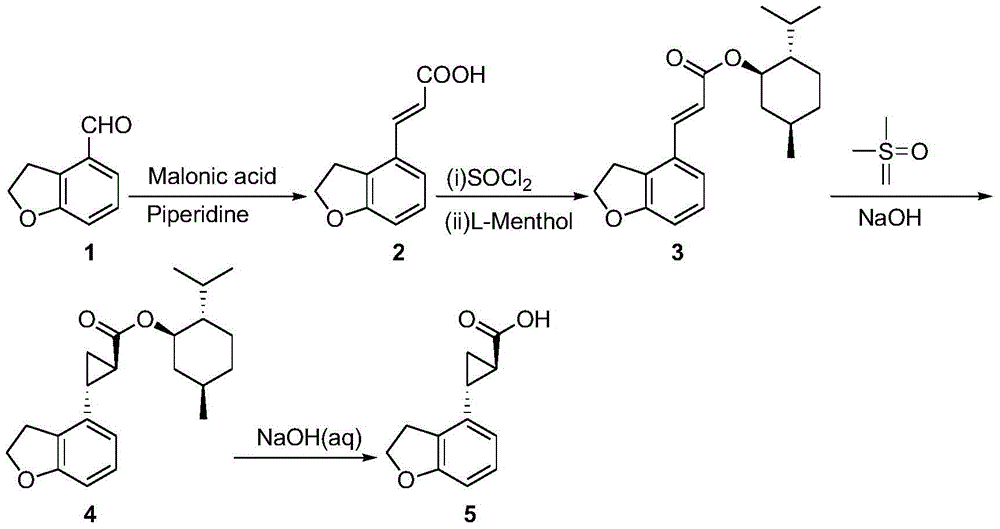
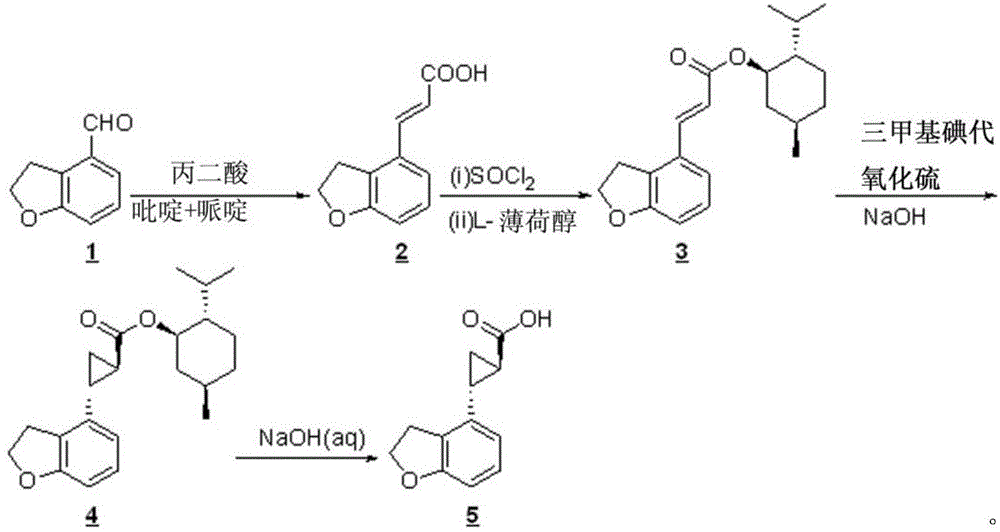
New preparation process of tasimeltion intermediate
A technology of tasimelteon and preparation process, which is applied in the field of preparation of synthesizing tasimelteon intermediates, and can solve problems such as inability to enlarge
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] (compound 2 Synthesis)
[0022] Add pyridine (1.3Kg) and piperidine (50g) into a 10L four-neck flask equipped with a thermometer, mechanical stirring, constant pressure dropping funnel, and tail gas absorption device, stir and heat up to 90-100°C, slowly add 2,3 - Dihydrobenzofuran-4-carbaldehyde (1.0 Kg), then malonic acid (1.5 Kg) was added. Stirring was continued at 90-100°C for 4 hours; then water (4.0 L) was added, and a pyridine / water mixture (2.5 L) was evaporated from the reaction flask under reduced pressure. After cooling to room temperature, the pH of the reaction solution was adjusted to 1 with 37% concentrated hydrochloric acid (4.5Kg), and a large amount of solids were precipitated. Filter, wash twice with 1% hydrochloric acid (3 L each time), and dry under vacuum at 40-50°C to obtain 2,3-dihydrobenzofuran-4-acrylic acid (1.1Kg), with a yield of 85% and a purity of 98%.
[0023] (compound 3 Synthesis)
[0024] compound 2 (1.0Kg), a mixture of toluene...
Embodiment 2
[0032] (compound 2 Synthesis)
[0033] Add pyridine (1.3Kg) and piperidine (50g) into a 10L four-neck flask equipped with a thermometer, mechanical stirring, constant pressure dropping funnel, and tail gas absorption device, stir and heat up to 90-100°C, slowly add 2,3 -Dihydrobenzofuran-4-carbaldehyde (1.0 Kg), then malonic acid (1.8 Kg) was added. Stirring was continued at 90-100°C for 4 hours; then water (4.0 L) was added, and a pyridine / water mixture (2.5 L) was evaporated from the reaction flask under reduced pressure. After cooling to room temperature, the pH of the reaction solution was adjusted to 1 with 37% concentrated hydrochloric acid (4.5Kg), and a large amount of solids were precipitated. Filter, wash twice with 1% hydrochloric acid (3 L each time), and dry under vacuum at 40-50°C to obtain the product (1.2Kg), with a yield of 93% and a purity of 98%.
[0034] (compound 3 Synthesis)
[0035] compound 2 (1.0Kg), a mixture of toluene (1.0kg) and pyridine (30g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com