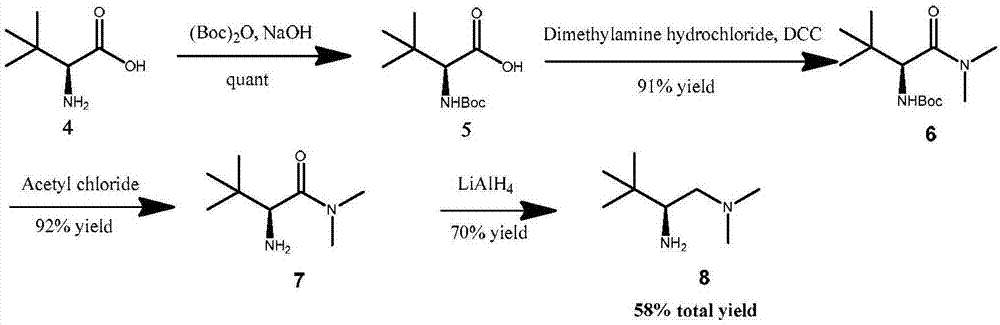
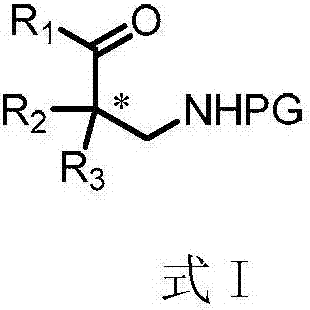
Chiral beta-amino acid derivative and preparation method thereof
A technology of derivatives and amino acids, which is applied in the field of chiral β-amino acid derivatives and their preparation, can solve problems such as the inability to truly meet the requirements of high-efficiency synthesis of β-amino acids, low selectivity, cumbersome process, etc., and reduce the cost of synthesis , high optical selectivity and high catalytic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Embodiment 1, N, the preparation of O-acetal
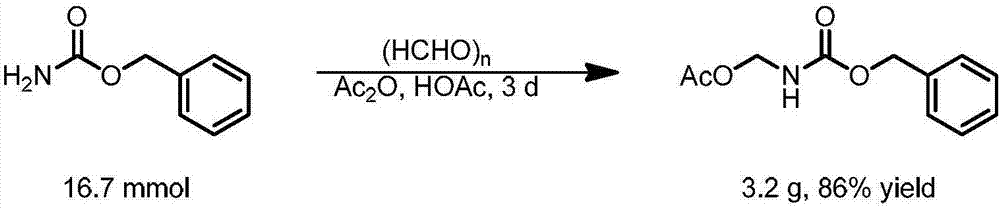
[0077] The present invention prepares N, O-acetal, is to use CbzNH 2 As the starting material, the N, O-acetal compound can be obtained through the condensation of amine and aldehyde and the protection of the hydroxyl group through Ac, which includes the following steps:
[0078] CbzNH 2 (Benzyl carbamate), paraformaldehyde are mixed at a molar ratio of 1:1.1, acetic acid and acetic anhydride are mixed at a volume ratio of 1:3 as a reaction solvent, stirred at 60°C for 1 day, and unreacted acetic anhydride is removed and acetic acid to obtain the corresponding very stable Cbz-protected amino N, O-acetal compound, the specific reaction equation is as figure 2 shown;
[0079]
[0080] The structure is confirmed as follows: 1 H NMR (400MHz, CDCl 3 )δ7.44–7.27(m,5H),5.97(s,1H),5.21(t,J=7.9Hz,2H),5.14(s,2H),2.06(s,3H). 13 C NMR (101MHz, CDCl 3 )δ171.29, 155.81, 135.74, 128.26, 128.01, 127.98, 66.92, 66.47, 20.58.
Embodiment 2
[0081] The preparation of embodiment 2, β-amino acid ester
[0082] Prepare according to the reaction equation shown below:
[0083]
[0084] Add ethyl 2-methylacetoacetate (50mmol) and N,O-acetal (75mmol) into the reactor, then dissolve compound 8 (2.5mmol) in 1ml of dichloromethane, drop trifluoro Methanesulfonic acid (2.5mmol), after dichloromethane was distilled off, the catalyst was added to the reactor, and finally m-nitrobenzoic acid (12.5mmol) was added, heated to 60°C, and the reaction was completed after stirring for 3 days, and the target was obtained by column chromatography. Product β-amino acid ester 83%, 99% ee.
[0085] The NMR data for the confirmation of the structure of β-amino acid ester are as follows: 1 H NMR (400MHz, CDCl 3 )δ7.42–7.26(m,5H),5.30(s,1H),5.07(s,2H),4.17(dt,J=6.9,4.1Hz,2H),3.68–3.48(m,2H),2.18 (s,3H),1.40(s,3H),1.24(t,J=7.1Hz,3H). 13 C NMR (101MHz, CDCl 3 )δ205.68, 171.72, 156.63, 136.57, 128.64, 128.26, 128.20, 66.96, 61.92, 60.60...
Embodiment 3
[0087] Prepare as follows according to the following reaction equation:
[0088]
[0089] Add ethyl cyclohexanone (50mmol) and N,O-acetal (75mmol) into the reactor, then add the chiral primary tertiary diamine organic small molecule catalyst (2.5mmol) shown in formula 5-1 with 1ml Dichloromethane was dissolved, and trifluoromethanesulfonic acid (2.5mmol) was added dropwise under a low-temperature ice bath. After dichloromethane was evaporated, the catalyst was added to the reactor, and m-nitrobenzoic acid (12.5mmol) was added at last, and heated to 60 ℃, the reaction was completed after stirring for 2 days, and the target product β-amino acid ester was obtained by column chromatography with 86% and >99% ee. The NMR data of its structural confirmation are as follows: 1 H NMR (400MHz, CDCl 3 )δ7.40–7.26(m,5H),5.44(d,J=19.0Hz,1H),5.13–4.99(m,2H),4.15(q,J=7.1Hz,2H),3.61(dd,J =13.8,7.8Hz,1H),3.43(dd,J=13.8,5.5Hz,1H),2.63–2.49(m,1H),2.49–2.35(m,2H),2.01(dt,J=9.6,6.1 Hz,1H),1....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com