Polycarbonate with optical activity
A polycarbonate, optically active technology, applied in the polycarbonate field, can solve the problems of the greenhouse effect, harsh reaction conditions, single product type, etc., and achieve the effects of broad industrial promotion value, mild reaction conditions and high optical selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
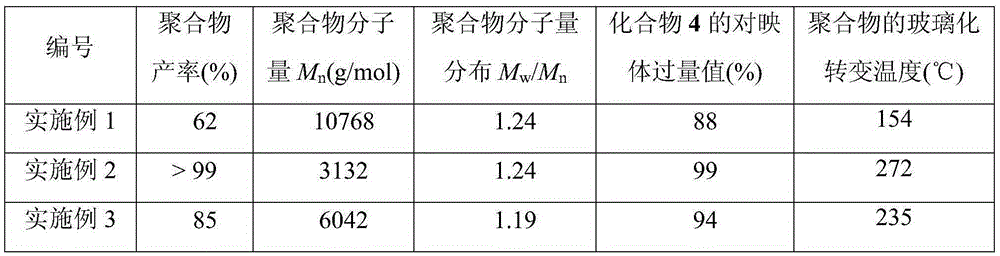
[0020] (1) Add chiral ligand A (Ar=Ph) 0.061 g, 0.1 mmol) and 2 mL toluene into a Schlenk reaction tube, and replace the air in the tube with nitrogen. Slowly add ZnEt dropwise at 0°C (ice-water bath) under nitrogen protection 2 (Organometallic; 0.2mL, 1.0M, 0.2mmol), then stirred at room temperature for 30min. Then add absolute ethanol (0.04mL, 1M, 0.04mmol), continue to stir at room temperature for 15min, then add 2mmol of 7-oxobicyclo[4.1.0]-3-heptene (1a), bubble with carbon dioxide, 30°C The reaction was stirred for 36h.
[0021] 40 mL of dichloromethane was added to the reaction system for dilution, the organic phase was washed with 1M hydrochloric acid (3×20 mL) and saturated sodium chloride solution (2×20 mL), and the organic phase was dried over anhydrous magnesium sulfate. Concentrate under reduced pressure to about 3 mL, add 40 mL of methanol to precipitate the polymer, filter, wash with methanol, and dry the product to constant weight to obtain a white solid poly...
Embodiment 2
[0025] (1) Add chiral ligand A (Ar=Ph) 0.061 g, 0.1 mmol) and 2 mL toluene into a Schlenk reaction tube, and replace the air in the tube with nitrogen. Slowly add ZnEt dropwise at 0°C (ice-water bath) under nitrogen protection 2 (Organometallic; 0.2mL, 1.0M, 0.2mmol), then stirred at room temperature for 30min. Then add absolute ethanol (0.04mL, 1M, 0.04mmol), continue to stir at room temperature for 15min, then add 2mmol of cyclopentene oxide (1b), bubble carbon dioxide, and stir at 30°C for 36h.
[0026] 40 mL of dichloromethane was added to the reaction system for dilution, the organic phase was washed with 1M hydrochloric acid (3×20 mL) and saturated sodium chloride solution (2×20 mL), and the organic phase was dried over anhydrous magnesium sulfate. Concentrate under reduced pressure to about 3 mL, add 40 mL of methanol to precipitate the polymer, filter, wash with methanol, and dry the product to constant weight to obtain 0.257 g of white solid polyoxycyclopentene carbo...
Embodiment 3
[0030] (1) Add chiral ligand A (Ar=Ph) 0.061 g, 0.1 mmol) and 2 mL toluene into a Schlenk reaction tube, and replace the air in the tube with nitrogen. At 0° C. (ice-water bath) under nitrogen protection, ZnEt2 (organometallic; 0.2 mL, 1.0 M, 0.2 mmol) was slowly added dropwise thereto, and stirred at room temperature for 30 min. Then add absolute ethanol (0.04mL, 1M, 0.04mmol), continue to stir at room temperature for 15min, then add 2mmol of 4,4-dimethyl-3,5,8-trioxobicyclo[5.1.0]octane (1c), carbon dioxide bubbles, stirred at 30°C for 36h.
[0031] 40 mL of dichloromethane was added to the reaction system for dilution, the organic phase was washed with 1M hydrochloric acid (3×20 mL) and saturated sodium chloride solution (2×20 mL), and the organic phase was dried over anhydrous magnesium sulfate. Concentrate under reduced pressure to about 3 mL, add 40 mL of methanol to precipitate the polymer, filter, wash with methanol, and dry the product to constant weight. 0.321 g (y...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com