A kind of optically active aie fluorescent material and preparation method of water-based polymer
A technology of fluorescent polymers and fluorescent materials, applied in the chemical industry, can solve the problems of no light emission and weak fluorescence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
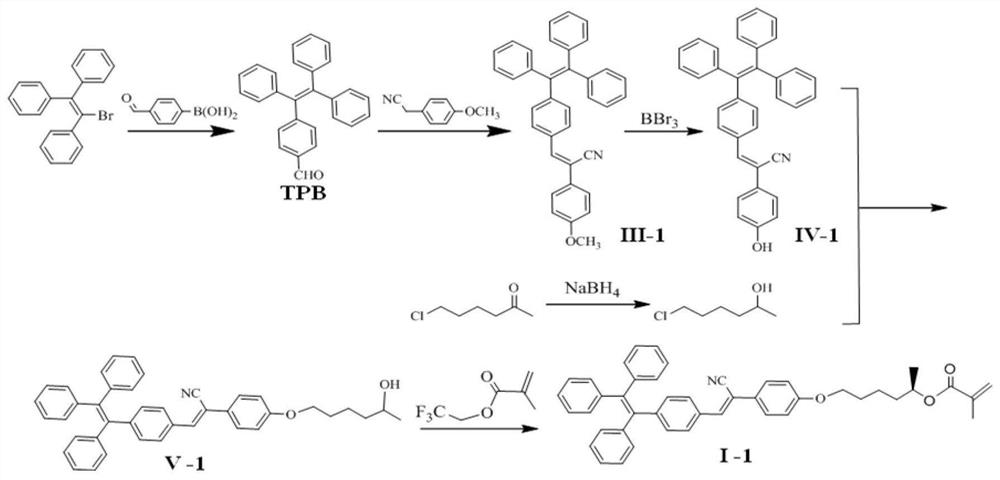
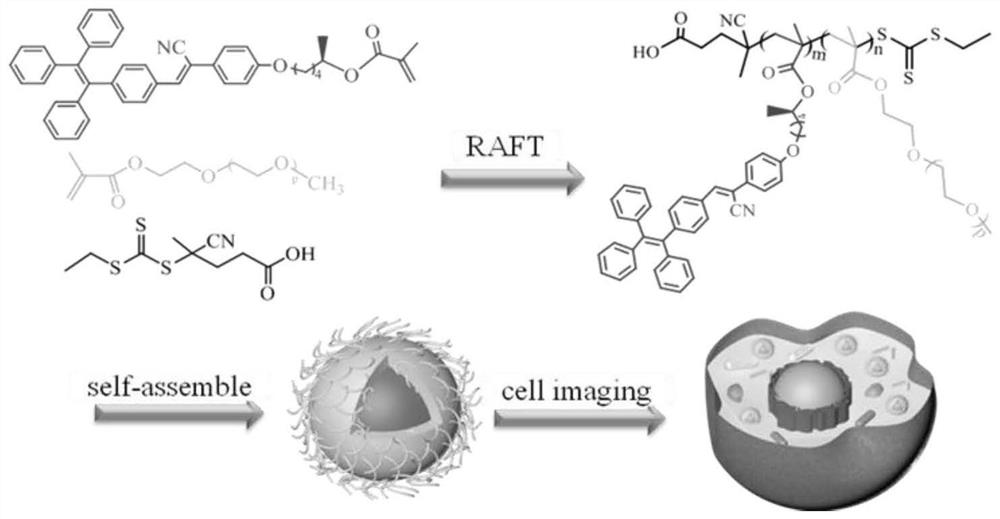
[0046] Example 1: Preparation of aqueous optically active fluorescent polymer (II-1), the synthetic route of which is as follows figure 1 and figure 2 shown, including:
[0047] Step 1: Triphenyl bromide (7.04mmol, 2.3581g), 4-formylbenzeneboronic acid (8.48mmol, 1.2647g), tetrabutylammonium bromide (0.704mmol, 0.2268g), 2mol / L carbonic acid Potassium aqueous solution (12.6 mL) and toluene (12.6 mL) were added to the reaction tube in turn, and stirred at room temperature for 0.5 h; tetrakis(triphenylphosphine)palladium(0)(8.22×10 -3 mmol, 9.5mg), the reaction tube was put into an oil bath at 90°C for 48h under nitrogen protection; after the reaction, deionized water was added, extracted with ethyl acetate, the organic layer was dried with anhydrous magnesium sulfate, filtered, After distillation under reduced pressure, the obtained crude product was separated and purified by silica gel chromatography (eluent: n-hexane / dichloromethane 2:1 volume ratio) to obtain 4-(1,2,2-tri...
Embodiment 2
[0061] Example 2: AIE properties of aqueous optically active fluorescent polymers
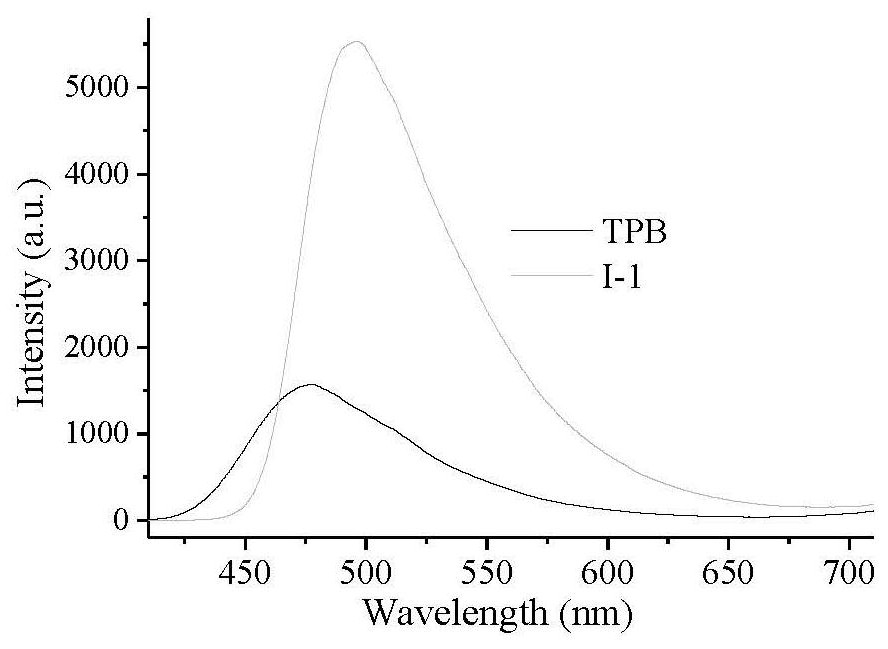
[0062] The solid fluorescence of optically active AIE fluorescent monomer I-1 was measured. Compared with the reported TPB fluorescent dyes, its fluorescence intensity was significantly enhanced, and the fluorescence emission wavelength increased from 475nm to 496nm, which had an obvious red shift (see image 3 ). with CDCl 3 as solvent, the obtained compounds IV-1, V-1, I-1, II-1 and II-2 1 The HNMR spectrum is as Figure 4 As shown, each compound appeared its own characteristic peak, indicating that each compound was successfully prepared. Weigh 10 mg of water-based optically active fluorescent polymer II-1 and dissolve them in 4 mL of mixed solutions of deionized water and tetrahydrofuran in different volume ratios, and measure the fluorescence emission intensity (see Figure 5 ). In the aqueous solution, there is an obvious fluorescence emission peak at 495 nm, while there is almost no...
Embodiment 3
[0063]Example 3: Optical properties of optically active AIE fluorescent monomer I-1 and its amphiphilic polymer
[0064] Weigh a certain amount of optically active AIE fluorescent monomer I-1 and its water-based polymer, dissolve it in 20 mL of acetone (concentration is 10.0 mg / mL), prepare a uniform and transparent solution, add it to a 20 cm long optical rotation tube, and measure the fluorescent monomer. and the specific optical rotation of the polymer. The calculated specific optical rotations of the AIE fluorescent monomer I-1 and its aqueous polymer II-2 are [α] 20 D = -9.67° and [α] 20 D =-3.74°.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific rotation | aaaaa | aaaaa |
| specific rotation | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com