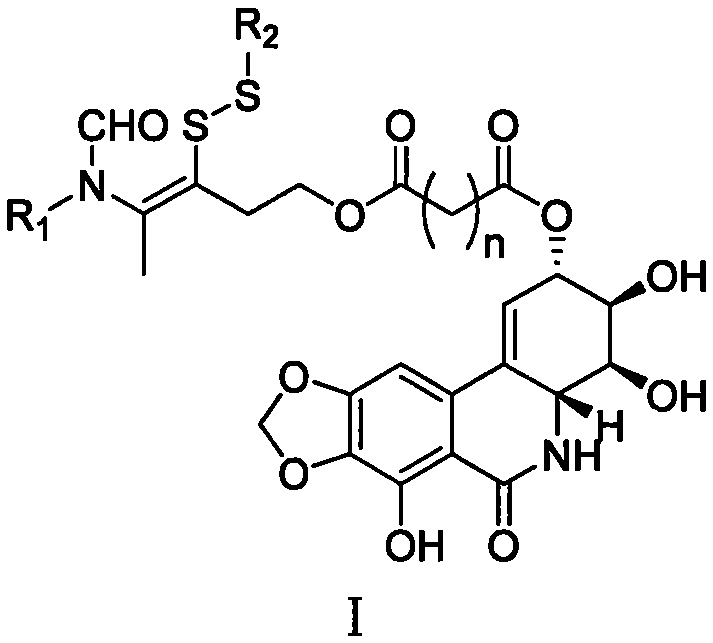
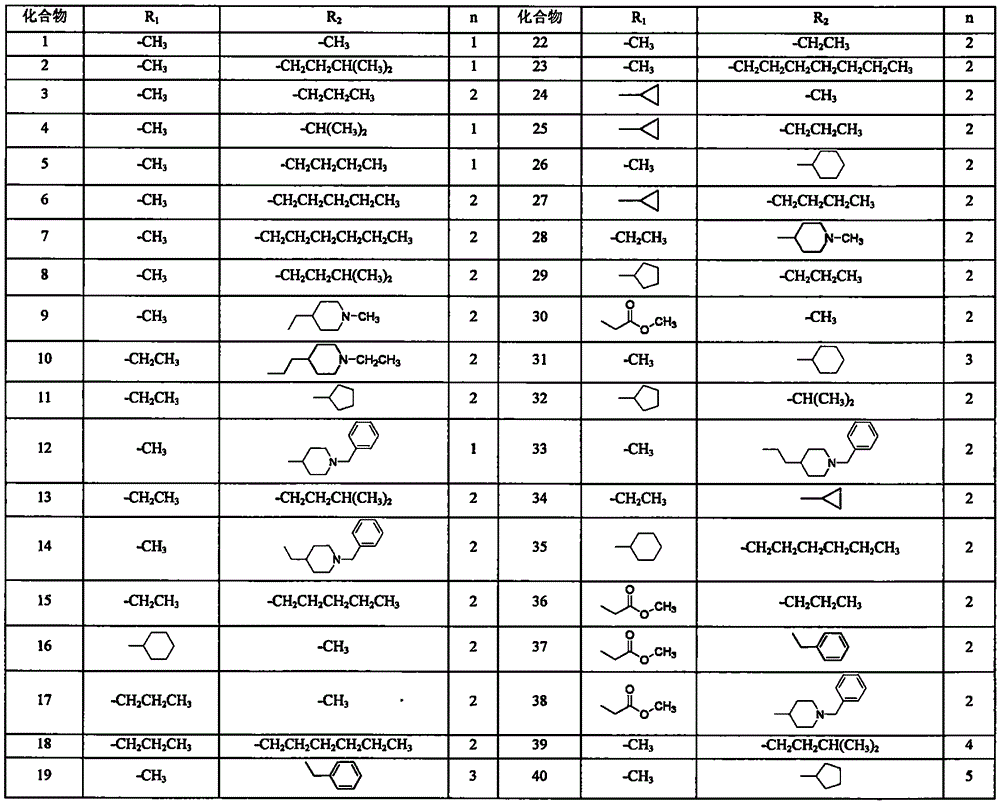
Narciclasine derivative, and preparation and application thereof in preparation of antitumor drugs
A technology of narciscycline and its derivatives, applied in the field of medicine, can solve problems such as poor selectivity and narrow therapeutic window
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Example 1: (3aS, 3bR, 12S, 12aR)-6,12-dihydroxy-2,2-dimethyl-3b,4,12,12a-tetrahydrobis([1,3]dioxo)[ 4,5-c: Preparation of 4′,5′-j]phenanthridin-5(3aH)-one
[0059]
[0060] Take 92 mg (0.30 mmol) of narciscycline, 100 mg (0.96 mmol) of 2,2-dimethoxypropane and 60 mg (0.35 mmol) of p-toluenesulfonic acid, dissolve them in 5 ml of anhydrous N , in N-dimethylformamide, stirred and reacted at room temperature 25° C. for 12 hours, and monitored the reaction by thin-layer chromatography. After the reaction is complete, add 0.8 milliliters of pyridine dropwise to the reaction mixture to quench the reaction, and continue to stir for 1 hour, then add 30 milliliters of water to the resulting mixture, a precipitate is precipitated, filter, wash the filter cake with water, and place the filter cake in a vacuum After drying in a drying oven at 55°C for 6 hours, 77 mg of a light brown powdery product was obtained, with a yield of 74%. 1 H NMR (500MHz, deuterated dimethyl sulfoxi...
Embodiment 2
[0061] Embodiment 2: the preparation of ethyl sodium thiosulfate
[0062]
[0063]Take 2.725 g (25 mmol) of bromoethane and dissolve it in 24 ml of ethanol to obtain a clear solution; take another 3.16 g (20 mmol) of anhydrous sodium thiosulfate and dissolve it in 18 ml of water. Mix ethyl bromide ethanol solution and sodium thiosulfate aqueous solution, stir and reflux at 110°C for 15 hours, evaporate the solvent, wash the crude product with ether, and dry to obtain 3.15 g of white crystals, with a yield of 96%, which is ready for use in the next reaction .
Embodiment
[0064] Embodiment: 3: the preparation of n-propyl sodium thiosulfate
[0065]
[0066] With reference to the molar ratio of feeding described in Example 2, reaction conditions and post-treatment mode: get 3.075 grams (25 mmoles) of 1-bromopropane to react to obtain 3.48 grams of white crystals, with a yield of 95%, and directly throw it into the next step for subsequent reactions.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com