Preparation method of galactose type salidroside and derivative thereof
A technology of salidroside and galactoside, which is applied in the field of sugar engineering, can solve the problems of time-consuming and cumbersome, single product, etc., and achieve the effects of simple operation, cheap substrate, and reduced production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0052] The preparation method of galactosyl salidroside and derivatives thereof comprises the steps of:
[0053](1) A reaction system with a lactose concentration of 1M, a p-hydroxyphenylethanol concentration of 0.25M, and a β-galactosidase concentration of 0.4mg / mL was prepared using a phosphate buffer solution with a concentration of 50mM and a pH of 7.0;
[0054] The β-galactosidase in the step (1) is the β-galactosidase whose amino acid sequence is shown in SEQ ID NO.1 as described in Chinese patent document CN1786170A (application number 200510044897.9);
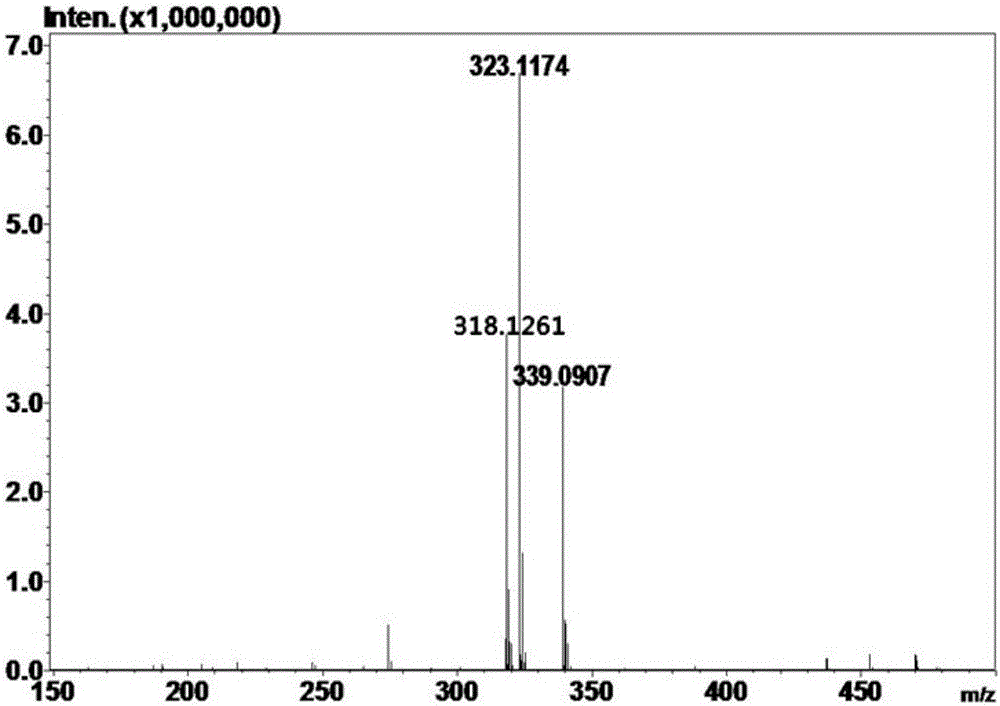
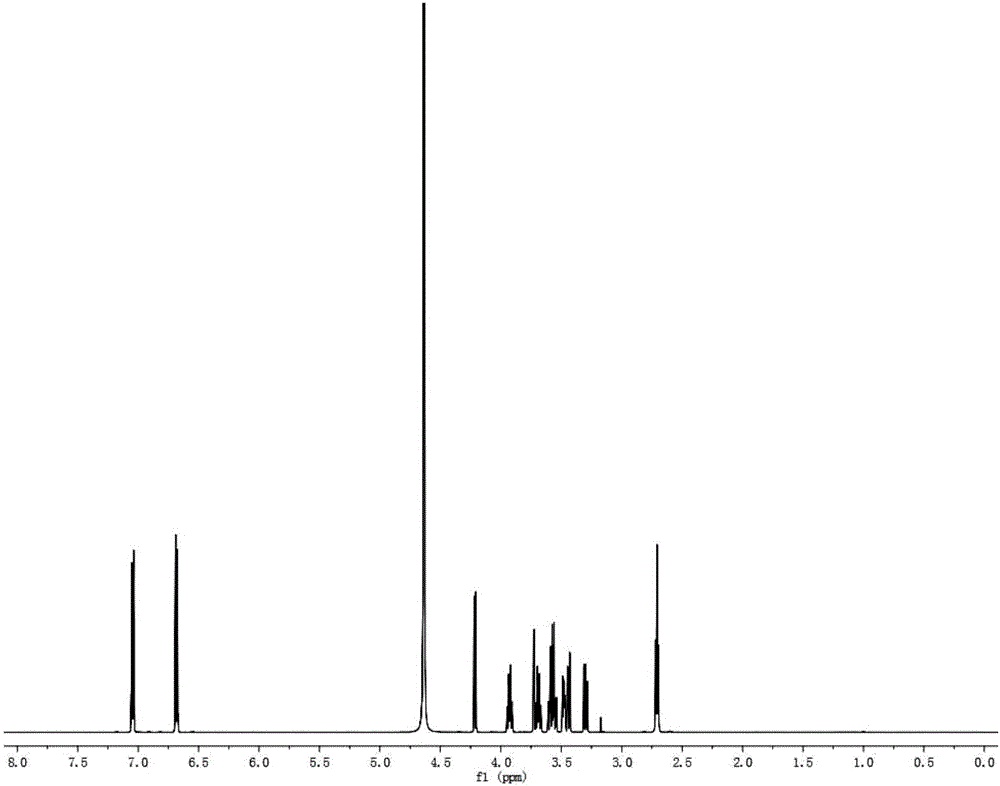
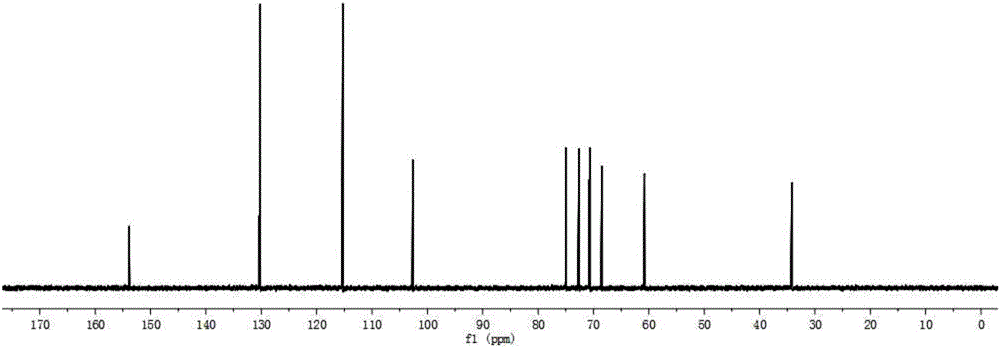
[0055] (2) React the reaction system prepared in step (1) at 50°C for 5 minutes, boil at 100°C for 10 minutes to terminate the reaction, centrifuge at 10,000 rpm for 20 minutes, and take the supernatant; HPLC analysis, the The total yield of galactosyl-salidroside and its derivatives produced by the reaction was 50.0%, of which galactosyl-salidroside accounted for 39.4%, and the two derivatives accounted for 10.6%.
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com