A kind of polypeptide and its use and preparation method
A technology of pramlintide and amino acids, which is applied to the preparation method of peptides, chemical instruments and methods, and medical preparations containing active ingredients, etc., which can solve the problems of high frequency of administration, unacceptable, short half-life, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0285] The preparation of the Fmoc-Tyr(tBu)-MBHAResin whose degree of substitution is about 0.25mmol / g in embodiment 1
[0286] Take 2.5kg of RinkAmideMBHAResin resin (1.0mol in total) with a degree of substitution of 0.4mmol / g, add it to a solid-phase reaction column, wash it twice with DMF, swell the resin with DMF for 30 minutes, and remove it twice with 20% DBLK solution Fmoc, 10 minutes each time, and then the resin was washed 6 times with DMF. Fmoc-Tyr(tBu)-OH (0.8eq, 0.8mol, g), HOBt (0.96eq, 0.96mol, 130g) and DIPCDI (0.96eq, 0.96mol, 121g) were dissolved in DCM with a volume ratio of 1:1 The solution was mixed with DMF, added to a solid-phase reaction column, and reacted at room temperature for 2 hours. After the reaction, wash twice with DMF and twice with DCM. Then add a mixture of pyridine (5eq, 5mol, 396g) and acetic anhydride (5eq, 5mol, 505g) to seal the resin for 6h (add appropriate DMF to make it stir and disperse evenly). After washing 4 times with DMF and...
Embodiment 2
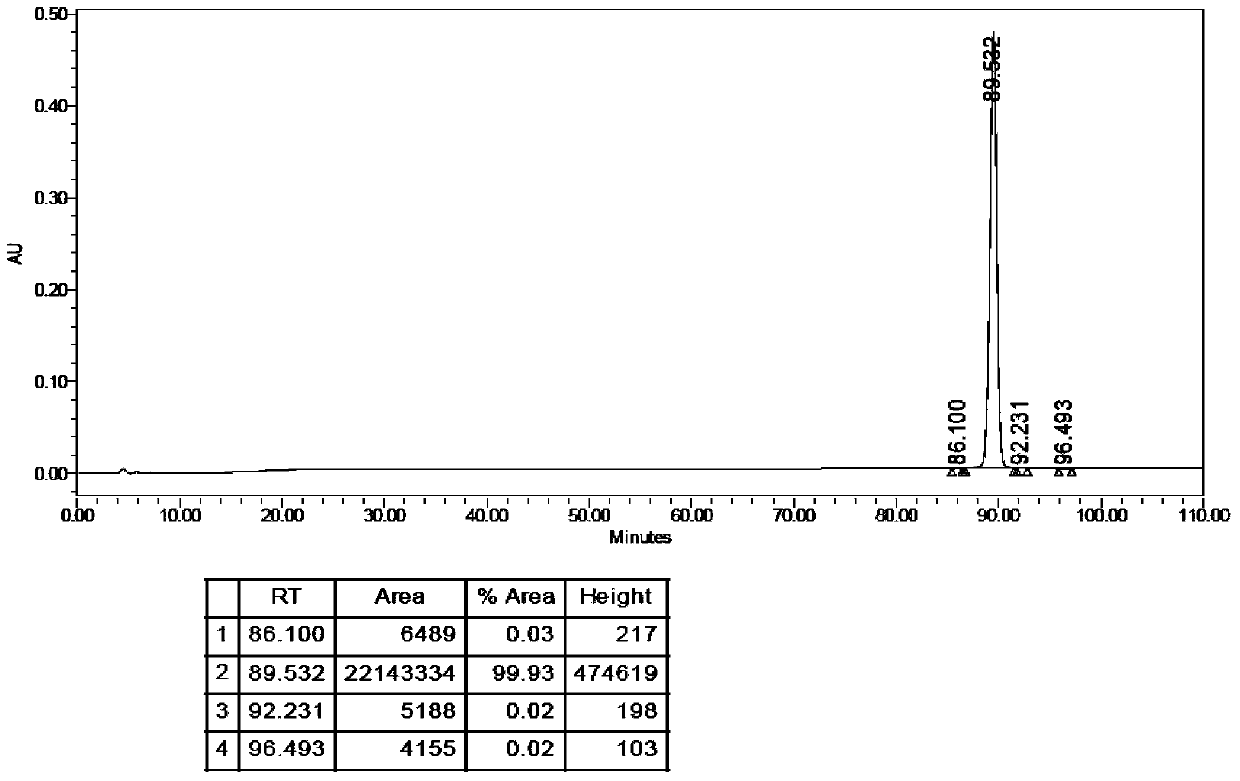
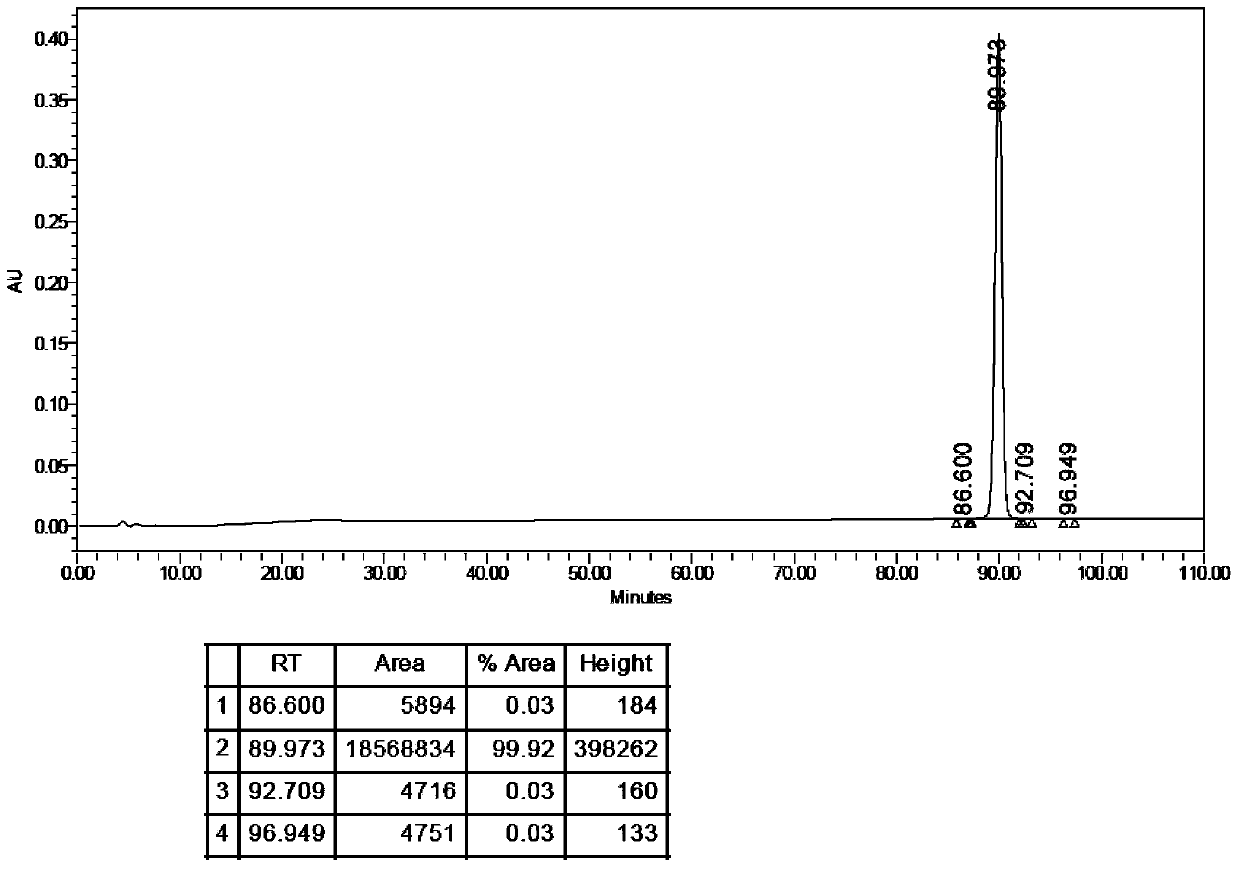
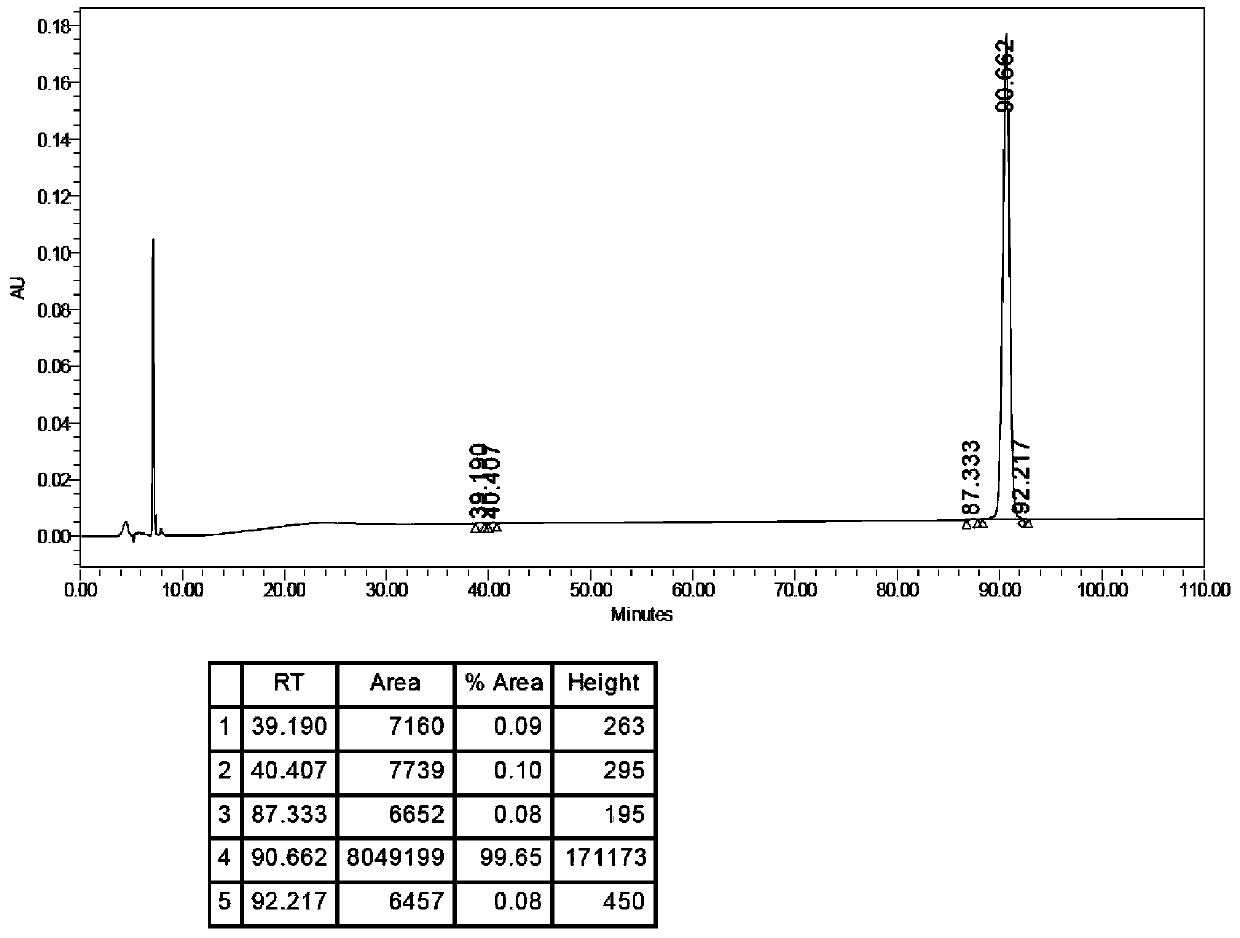
[0287] Embodiment 2 stearic acid-(γGlu)-[Lys 1 Preparation of side chain]-pramlintide
[0288] 2.1 Coupling of amino acids
[0289] Get 398g, 0.1mol Fmoc-Tyr(tBu)-MBHAResin resin prepared in Example 1 is added in the solid-phase reaction column, washed 2 times with DMF, after 30 minutes with DMF swelling resin, adopt 20% DBLK solution to remove twice Fmoc, 10 minutes each time, and then the resin was washed 6 times with DMF. Fmoc-Thr(tBu)-OH (5eq, 0.5mol, 199g), HOBt (6eq, 0.6mol, 81g) and DIPCDI (6eq, 0.6mol, 76g) were dissolved in DCM and DMF with a volume ratio of 1:1 and mixed The solution was added to a solid-phase reaction column, and reacted at room temperature for 2 hours, and the resin was detected to be colorless and transparent with 5% ninhydrin / ethanol solution. After the reaction was completed, it was washed 3 times with DMF.
[0290] According to the above coupling method, according to the pramlintide peptide sequence, sequentially couple Fmoc-Asn 35 (Trt)-O...
Embodiment 3
[0302] Example 3 Lauric acid-(γGlu)-[Lys 1 Preparation of side chain]-pramlintide
[0303] 3.1 Coupling of amino acids
[0304] Get 398g, 0.1mol Fmoc-Tyr(tBu)-MBHAResin resin prepared in Example 1 is added in the solid-phase reaction column, washed 2 times with DMF, after 30 minutes with DMF swelling resin, adopt 20% DBLK solution to remove twice Fmoc, 10 minutes each time, and then the resin was washed 6 times with DMF. Fmoc-Thr(tBu)-OH (5eq, 0.5mol, 199g), HOBt (6eq, 0.6mol, 81g) and DIPCDI (6eq, 0.6mol, 76g) were dissolved in DCM and DMF with a volume ratio of 1:1 and mixed The solution was added to a solid-phase reaction column, and reacted at room temperature for 2 hours, and the resin was detected to be colorless and transparent with 5% ninhydrin / ethanol solution. After the reaction was completed, it was washed 3 times with DMF.
[0305] According to the above coupling method, according to the pramlintide peptide sequence, sequentially couple Fmoc-Asn 35 (Trt)-OH, F...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com