Additives for high-purity copper electrolytic refining, method for producing high-purity copper, and high-purity electrolytic copper
A copper electrolytic refining, high-purity technology, applied in the direction of instruments, optics, photography, etc., can solve the problems of chlorine and silver shortage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
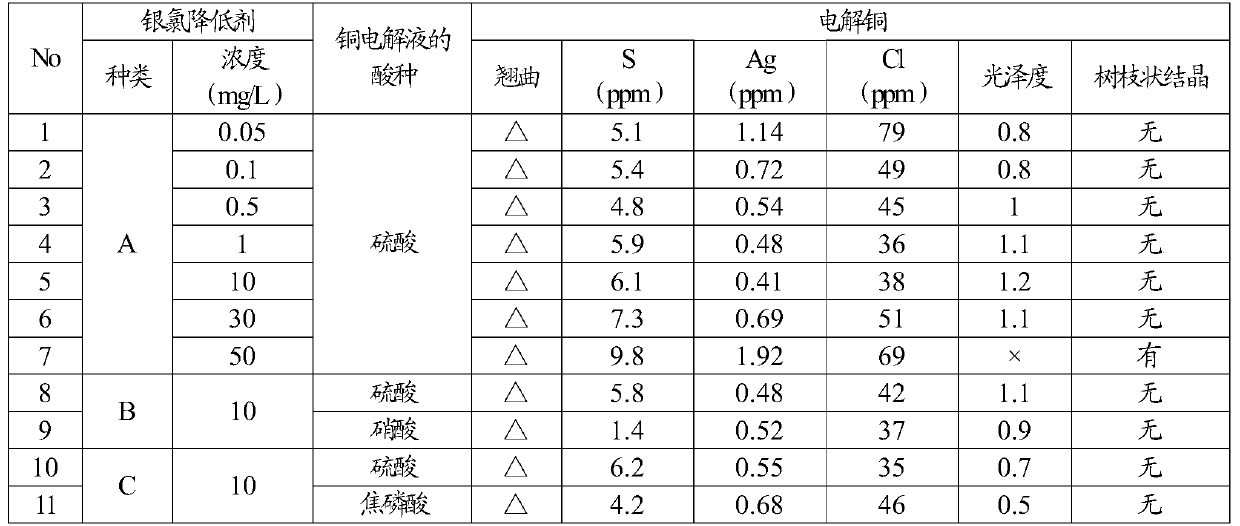
[0051] Using the silver chloride reducing agent (A, B, C) constituting the additive of the present embodiment, an aqueous copper sulfate solution and an aqueous copper nitrate solution adjusted to an acid concentration of 50 g / L, a copper concentration of 50 g / L, and a chloride ion concentration of 100 mg / L were used. Or a copper pyrophosphate aqueous solution is used as a copper electrolytic solution, and the above-mentioned silver chloride reducing agent is added in the mode of the concentration shown in Table 1 in this copper electrolytic solution. In addition, electrolytic copper having a sulfur concentration of 5 mass ppm and a silver concentration of 8 mass ppm was used for the anode, and a SUS316 plate was used as the cathode substrate. Set the current density to 200A / m 2 , copper electrolysis was carried out at a bath temperature of 30° C. for 5 days, and the concentration of the silver chloride reducing agent was measured by HPLC (high performance liquid chromatograph...
Embodiment 2
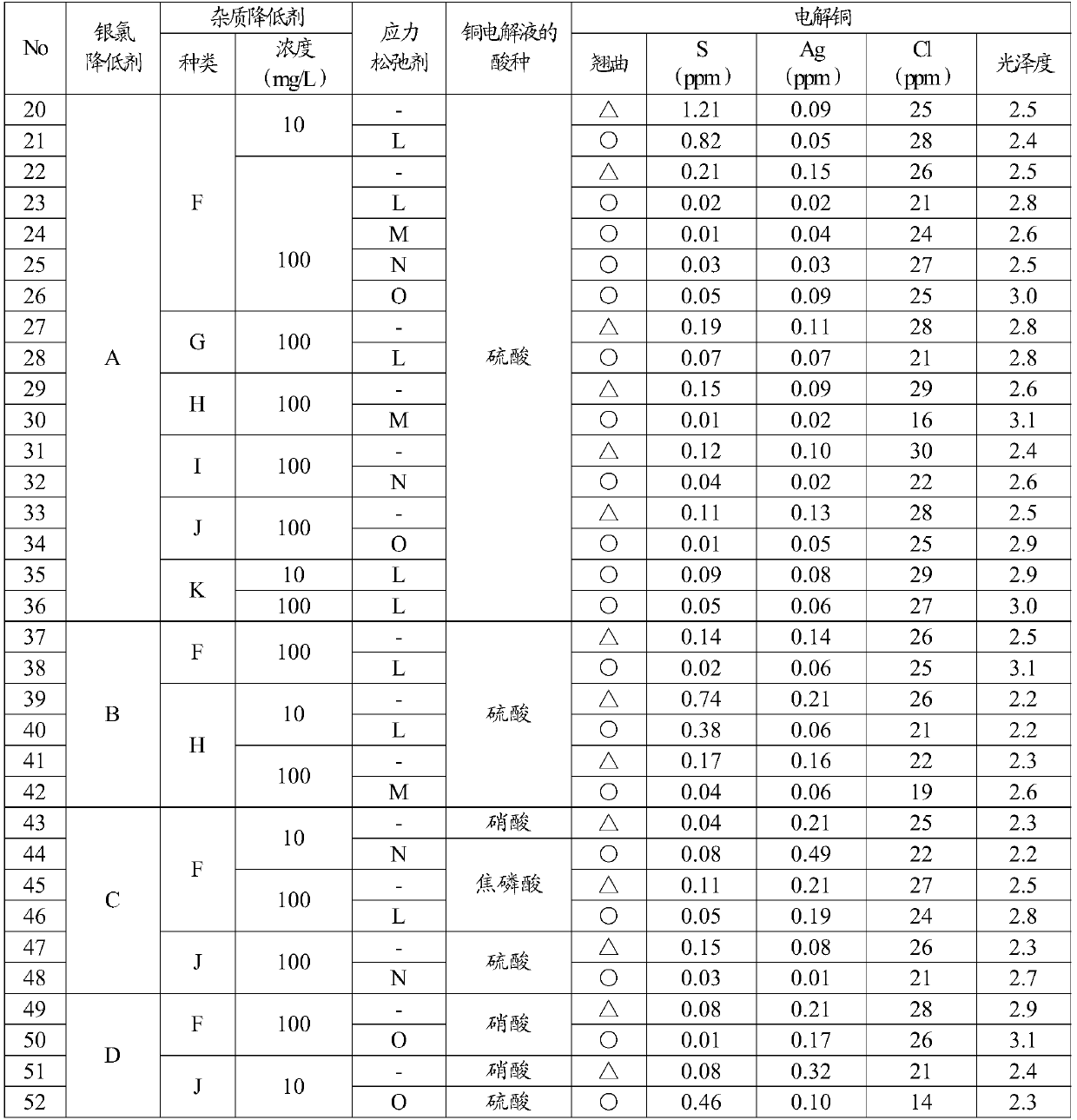
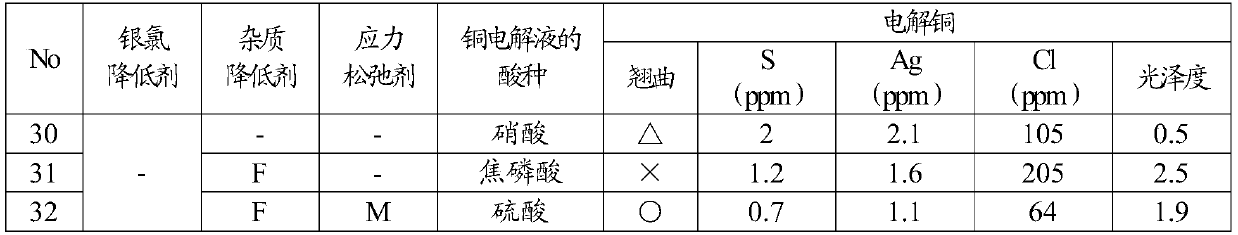
[0061]As shown in Table 2, together with the silver chloride reducing agent (A, B, C) of Example 1 and other silver chloride reducing agents D (5-phenyl-1H-tetrazole), the impurity reducing agent (F, G , H, I, J, K) are added to the copper electrolyte, and in addition, a part of the impurity reducing agent and / or stress relaxation agent (L, M, N, O) is used together with the silver chloride reducing agent. The added concentration of the silver chloride reducing agent was 10 mg / L, the added concentration of the impurity reducing agent was set to 10 mg / L, 100 mg / L, and when the stress relaxing agent was used, the concentration of the stress relaxing agent was set to 10 mg / L. Acid concentration in copper electrolytic solution, copper concentration, chloride concentration, and other electrolytic conditions Copper electrolytic refining was performed under the same conditions as in Example 1 to produce electrolytic copper. Impurity reducing agents (F to K) and stress relaxants (L to...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
| gloss | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com