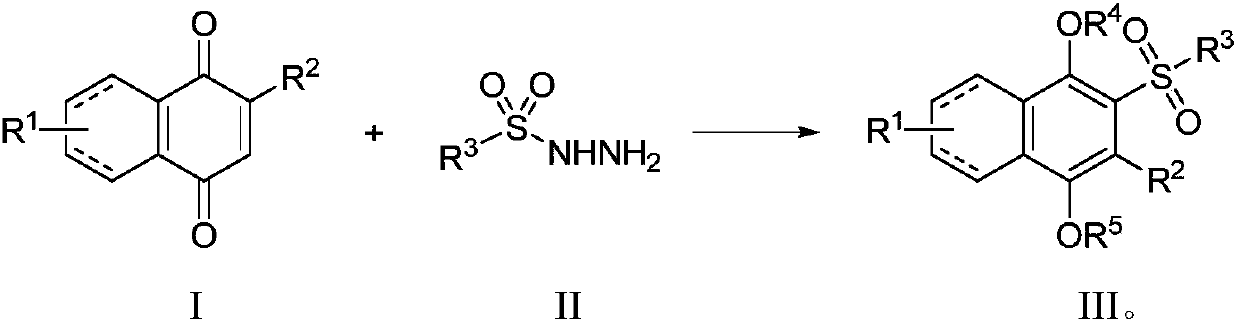
A kind of preparation method of sulfonyl hydroquinone compound
A technology for sulfonyl hydroquinone and compounds, which is applied in the field of preparation of sulfonyl hydroquinone compounds, to achieve the effects of increasing yield and simplifying the preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
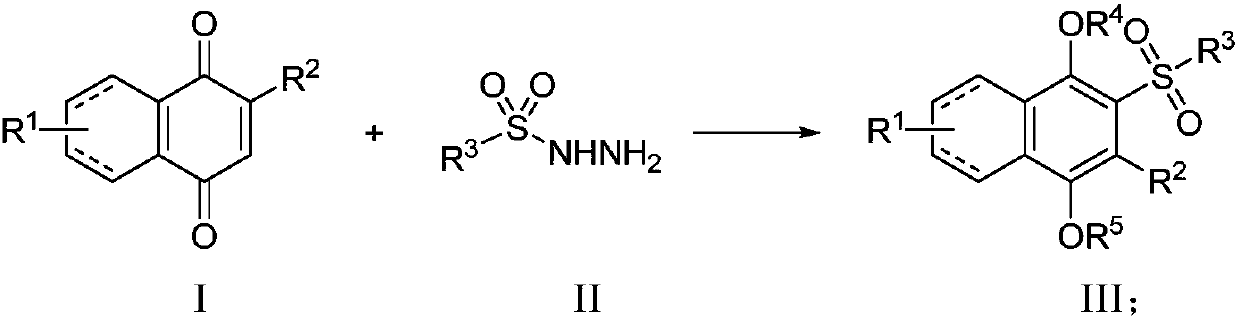
[0062] Embodiment 1 prepares 2-p-toluenesulfonyl-1,4-diphenol (3)
[0063]
[0064] A mixture of benzoquinone 1 (54 mg, 0.5 mmol), p-toluenesulfonyl hydrazide 2 (140 mg, 0.75 mmol) was dissolved in H 2 O (5.0 mL), the reaction mixture was reacted at room temperature for 12 hours, and the reaction was completed. The reaction was extracted three times with EtOAc, the combined organic phases were dried over anhydrous sodium sulfate, filtered, and the organic solvent was distilled off under reduced pressure. The residue was separated and purified by silica gel column chromatography to obtain 2-p-toluenesulfonyl-1,4-diphenol 3 with a yield of 67%. 1 H NMR (500MHz, CD 3 OD): δ7.83(d,J=6.8Hz,2H),7.40–7.29(m,3H),6.99–6.85(m,1H),6.74(dd,J=8.8,1.6Hz,1H),2.40 (s,3H). 13 C NMR (126MHz, CD 3 OD) δ149.94, 148.57, 144.18, 138.52, 129.00, 127.68, 126.08, 122.68, 118.19, 113.86, 20.11.
Embodiment 2
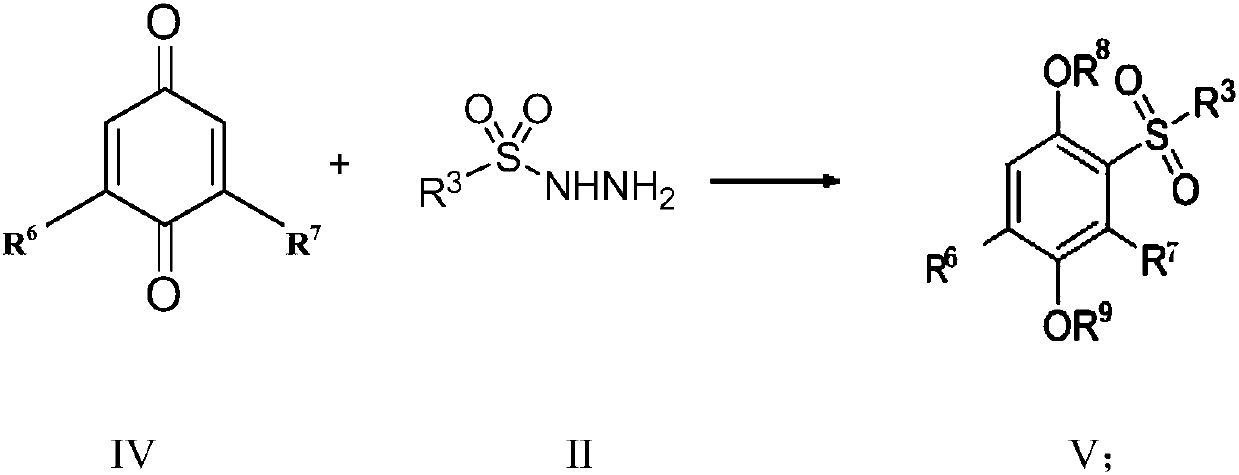
[0065] Example 2 Preparation of 2-benzenesulfonyl-1,4-diphenol (5)
[0066]
[0067] A mixture of benzoquinone 1 (54mg, 0.5mmol), benzenesulfonylhydrazide 4 (130mg, 0.75mmol) was dissolved in H 2 O (5.0 mL), the reaction mixture was reacted at room temperature for 12 hours, and the reaction was completed. The reaction was extracted three times with EtOAc, the combined organic phases were dried over anhydrous sodium sulfate, filtered, and the organic solvent was distilled off under reduced pressure. The residue was separated and purified by silica gel column chromatography to obtain 2-benzenesulfonyl-1,4-diphenol 5 with a yield of 65%. 1 H NMR (500MHz, CD 3 OD) δ7.96(d, J=7.9Hz, 2H), 7.61(t, J=7.4Hz, 1H), 7.53(t, J=7.6Hz, 2H), 7.40(d, J=2.8Hz, 1H ),6.95(dd,J=8.8,2.9Hz,1H),6.76(d,J=8.8Hz,1H). 13 C NMR (126MHz, CD 3 OD)δ149.95, 148.67, 141.41, 132.96, 128.48, 127.63, 125.83, 122.91, 118.24, 113.97.
Embodiment 3
[0068] Example 3 Preparation of 2-(2-bromobenzenesulfonyl)-1,4-diphenol (7)
[0069]
[0070] A mixture of benzoquinone 1 (54mg, 0.5mmol), 2-bromo-p-toluenesulfonylhydrazide 6 (190mg, 0.75mmol) was dissolved in H 2 O (5.0 mL), the reaction mixture was reacted at room temperature for 12 hours, and the reaction was completed. The reaction was extracted three times with EtOAc, the combined organic phases were dried over anhydrous sodium sulfate, filtered, and the organic solvent was distilled off under reduced pressure. The residue was separated and purified by silica gel column chromatography to obtain 2-(2-bromobenzenesulfonyl)-1,4-diphenol 7 with a yield of 60%. 1 H NMR (500MHz, CD 3 OD) δ8.36(d, J=7.9Hz, 1H), 7.71(d, J=7.9Hz, 1H), 7.60(t, J=7.6Hz, 1H), 7.50(dd, J=14.0, 6.9Hz ,1H),7.46(d,J=2.9Hz,1H),6.97(dd,J=8.8,2.9Hz,1H),6.72(d,J=8.8Hz,1H). 13 C NMR (126MHz, CD 3 OD)δ149.53, 148.76, 140.21, 134.81, 134.20, 132.18, 127.13, 124.38, 123.06, 119.94, 117.77, 115.83.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com