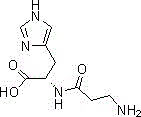
Synthetic method of L-carnosine
A synthesis method and carnosine technology, applied in the field of L-carnosine synthesis, can solve the problems of difficulty in meeting high-quality requirements of products, difficulty in industrialized production, and high solvent consumption, reducing the generation of by-products, shortening reaction steps, and shortening reaction steps. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
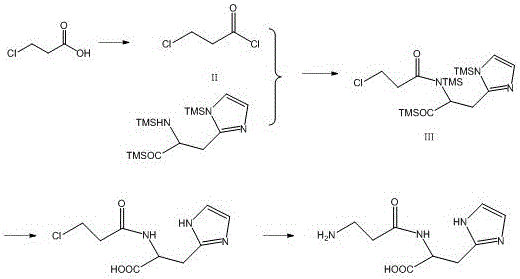
Embodiment 1
[0035] Preparation of 3-chloropropionyl chloride (II).
[0036] In a 1000ml reaction bottle, add 52.6g 3-chloropropionic acid (0.485mol), 500ml toluene, 72.0g triphosgene (0.485mol), slowly raise the temperature to 25°C, keep it warm for 8h, the material is a transparent liquid, and then recover toluene under reduced pressure, Evaporate to dryness to obtain 60.7 g of product (II), the yield is 98.5%. Add 500 ml of chloroform, stir and dissolve at room temperature, and obtain a light yellow transparent liquid, which is used for the following condensation reaction.
Embodiment 2
[0038] Preparation of 3-chloropropionyl chloride (II).
[0039] In a 500ml reaction bottle, add 26.3g 3-chloropropionic acid (0.242mol), 250ml toluene, 57.7g thionyl chloride (0.484mol), slowly raise the temperature to 20°C, keep it warm for 10h, the material is a transparent liquid, and then recover under reduced pressure Toluene was evaporated to dryness to obtain 30.1 g of product (II), with a yield of 98.0%. Add 250 ml of chloroform and stir to dissolve at room temperature to obtain a light yellow transparent liquid, which is used for the following condensation reaction.
Embodiment 3
[0041] Preparation of the amide product of L-histidine protected compound and 3-chloropropionyl chloride (Ⅲ)
[0042] In a 1000ml reaction flask, add 50g of trimethylsilane-protected L-histidine (0.323mol), add 600ml of chloroform and stir. At this time, the material is a transparent liquid. Cool down to 5°C, and then slowly add dropwise the The chloroform solution of 3-chloropropionyl chloride (0.484mol), the reaction temperature is 10-20°C, and the reaction is stirred overnight for 20h to obtain the product (Ⅲ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com