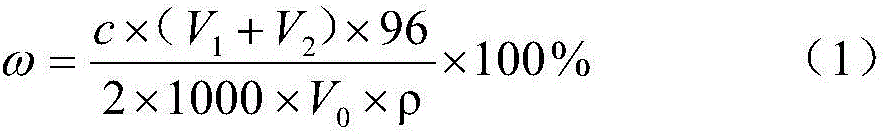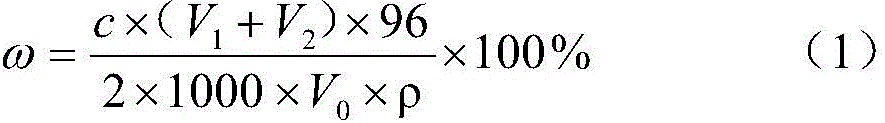Method for rapid determination of sulfate radical content in liquid phase in the process of production of potassium dihydrogen phosphate
A potassium dihydrogen phosphate and production process technology, applied in the field of analytical chemistry, can solve the problems of cumbersome steps, slow discoloration of the titration end point, not suitable for rapid analysis, etc., to achieve the effect of improving detection accuracy and sharp discoloration of the system
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Take 3mL, 3.5mL, 4mL, 4.5mL barium chloride standard solution into four 25mL colorimetric tubes respectively with a burette, then add 2mL acetic acid solution, 4mL stabilizer (50% ethanol + 30% chloroform + 20 % glyceryl stearate), 5-6 drops of sodium roseate indicator solution. Filter the sample, pipette 4mL filtrate into four colorimetric tubes, and shake the colorimetric tubes for about 30 seconds after the tubes are tightly plugged. The colors of the four colorimetric tubes are white, white, white, and red in sequence. (1) formula calculates and obtains that sulfate radical content is 0.85% in the sample, measures the sulfate radical content in the sample with gravimetric method simultaneously and is 0.81%, both errors are 0.04%. After the colorimetry, the pH value of the four colorimetric tubes was measured with a precision pH meter to be 2.5-2.6.
Embodiment 2
[0029] Take 3mL, 3.5mL, 4mL, 4.5mL barium chloride standard solution into four 25mL colorimetric tubes respectively with a burette, then add 3mL acetic acid solution, 4mL stabilizer (40% ethanol + 40% chloroform + 20 % methyl isobutyl ketone), 5-6 drops of sodium roseate indicator solution. Filter the sample, draw 2mL of the filtrate with a pipette and add them to the four colorimetric tubes. After tightening the stoppers, shake the colorimetric tubes for about 30 seconds. The colors of the four colorimetric tubes are all white. Take another colorimetric tube and add 5mL barium chloride standard solution, the other operations are the same as above, the color of the colorimetric tube is red. Calculate according to (1) formula and obtain the sulfate radical content in the sample to be 1.90%, measure the sulfate radical content in the sample with gravimetry simultaneously and be 1.84%, both error is 0.06%. After the colorimetry was finished, the pH value of the colorimetric tube...
Embodiment 3
[0031] Take 2mL, 2.5mL, 3mL, 3.5mL barium chloride standard solution into four 25mL colorimetric tubes respectively with a burette, then add 3mL acetic acid solution and 4mL stabilizer (40% ethanol + 40% acetone + 20% three ethanolamine), 5-6 drops of sodium rhodamine indicator solution. Filter the sample, draw 2mL of filtrate with a pipette and add them to four colorimetric tubes, and then shake the colorimetric tubes for about 30 seconds after tightening the stoppers. The colors of the four colorimetric tubes are white, white, red, and red (1) formula calculates and obtains that sulfate radical content is 1.10% in the sample, measures the sulfate radical content in the sample with gravimetric method simultaneously and is 1.07%, both error is 0.03%. After the colorimetry, the pH value of the colorimetric tubes in this group was measured by a precision pH meter at 2.0-2.1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com