Ziyuglycoside I solid dispersion body and preparation method thereof
A technology of solid dispersion and eucalyptus saponins, applied in the field of medicine, can solve problems such as difficult to obtain, poor efficacy of eucalyptus saponins I, and elevated blood cell levels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1 The preparation of Sangui saponin I solid dispersion of the present invention
[0024] Prescription: Burnet Saponin Ⅰ 1g, PVP K30 8g
[0025] Preparation method: Take the prescribed amount of burnet saponin Ⅰ, add 100ml of absolute ethanol and ultrasonically dissolve it, and obtain solution 1. Separately take the prescribed amount of PVP K30, add 4ml of absolute ethanol and sonicate until dissolved to obtain solution 2. Stir solution 2 on a constant temperature magnetic stirrer, and slowly add solution 1 to solution 2 with a dropper. After all solution 1 is added to solution 2, let the mixture stir for four hours on a constant temperature magnetic stirrer. Pour the mixed solution into an evaporating dish, put it on a 60°C water bath, evaporate to dryness, and grind the evaporated matter to obtain a solid dispersion.
Embodiment 2
[0026] Embodiment 2 Preparation of Burnet Saponin I Solid Dispersion of the Present Invention
[0027] Prescription: Burnet Saponin Ⅰ 5g, PVP K30 20g
[0028] Preparation method: Take the prescribed amount of burnet saponin Ⅰ, add 500ml of absolute ethanol and ultrasonically dissolve it, and obtain solution 1. Separately take the prescribed amount of PVP K30, add 20ml of absolute ethanol and sonicate until dissolved to obtain solution 2. Stir solution 2 on a constant temperature magnetic stirrer, and slowly add solution 1 to solution 2 with a dropper. After all solution 1 is added to solution 2, let the mixture stir for four hours on a constant temperature magnetic stirrer. Pour the mixed solution into an evaporating dish, put it on a 60°C water bath, evaporate to dryness, and grind the evaporated matter to obtain a solid dispersion.
Embodiment 3
[0029] Embodiment 3 Preparation of Burnet Saponin I Solid Dispersion of the Present Invention
[0030] Prescription: Burnet Saponin Ⅰ 10g, PVP K30 100g
[0031] Preparation method: Take the prescribed amount of Saponin I from Sangui, add 1000ml of absolute ethanol and ultrasonically dissolve it, and obtain solution 1. Take another prescribed amount of PVP K30, add 40ml of absolute ethanol and sonicate until dissolved to obtain solution 2. Stir solution 2 on a constant temperature magnetic stirrer, and slowly add solution 1 to solution 2 with a dropper. After all solution 1 is added to solution 2, let the mixture stir for four hours on a constant temperature magnetic stirrer. Pour the mixed solution into an evaporating dish, put it on a 60°C water bath, evaporate to dryness, and grind the evaporated matter to obtain a solid dispersion.
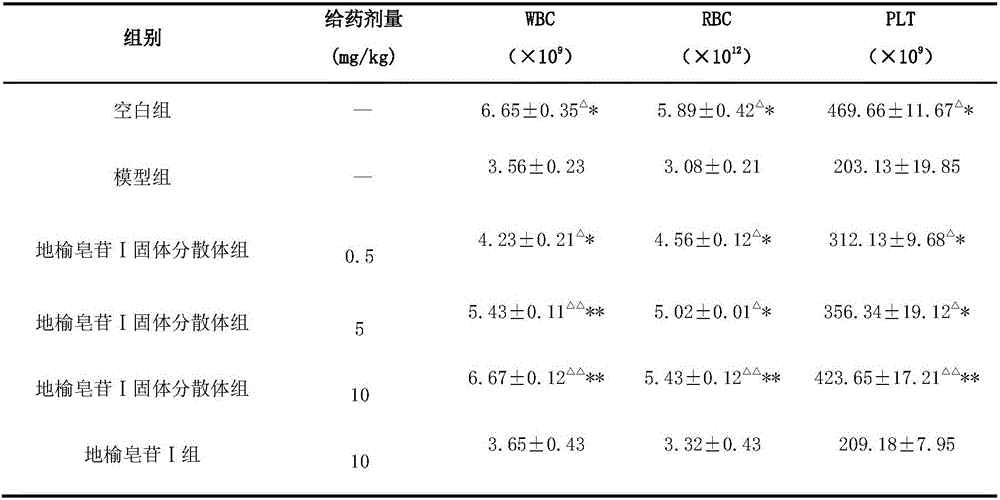
[0032] The beneficial effects of the present invention are demonstrated through experimental examples below.
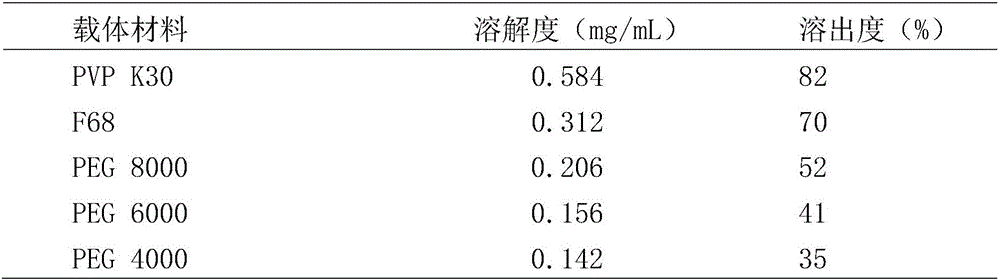
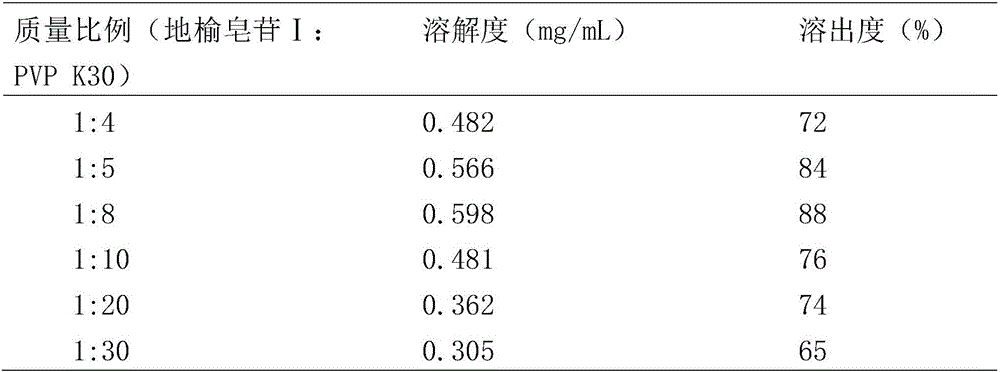
[0033] Dissolution test meth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com