Medicine controlled release transdermal micro needle system with magnetocaloric effect, preparation method and application
A technology of controlled drug release and magnetocaloric effect, applied in the field of medicine, can solve the problems of difficult control of drug release behavior, difficult absorption of macromolecular drugs, side effects in the gastrointestinal tract, etc., so as to improve the efficiency of drug delivery and release, and improve the effect of drug therapy. , easy to control the release effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
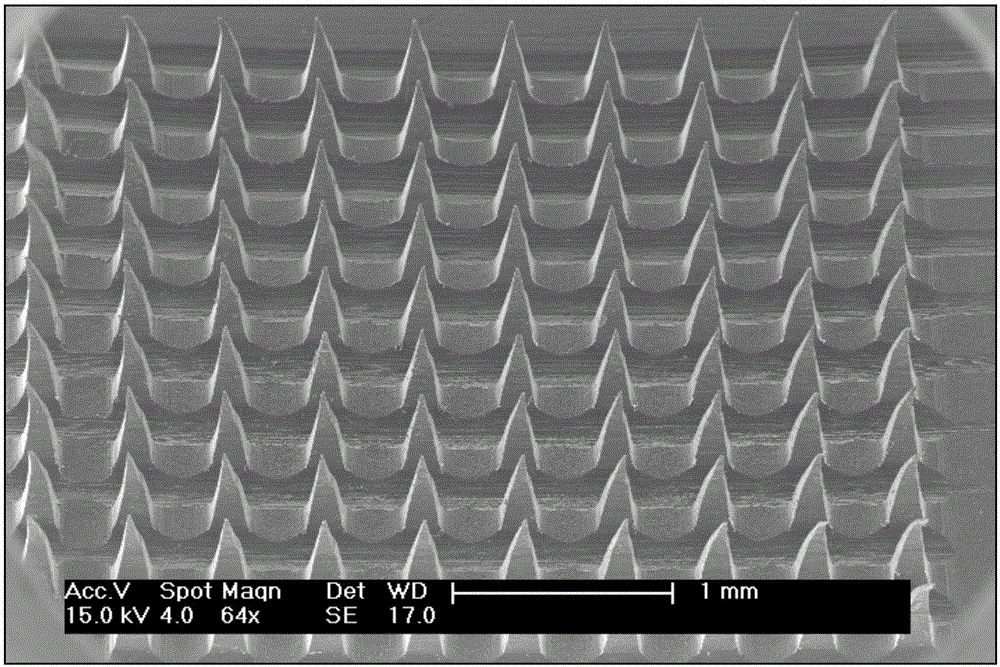
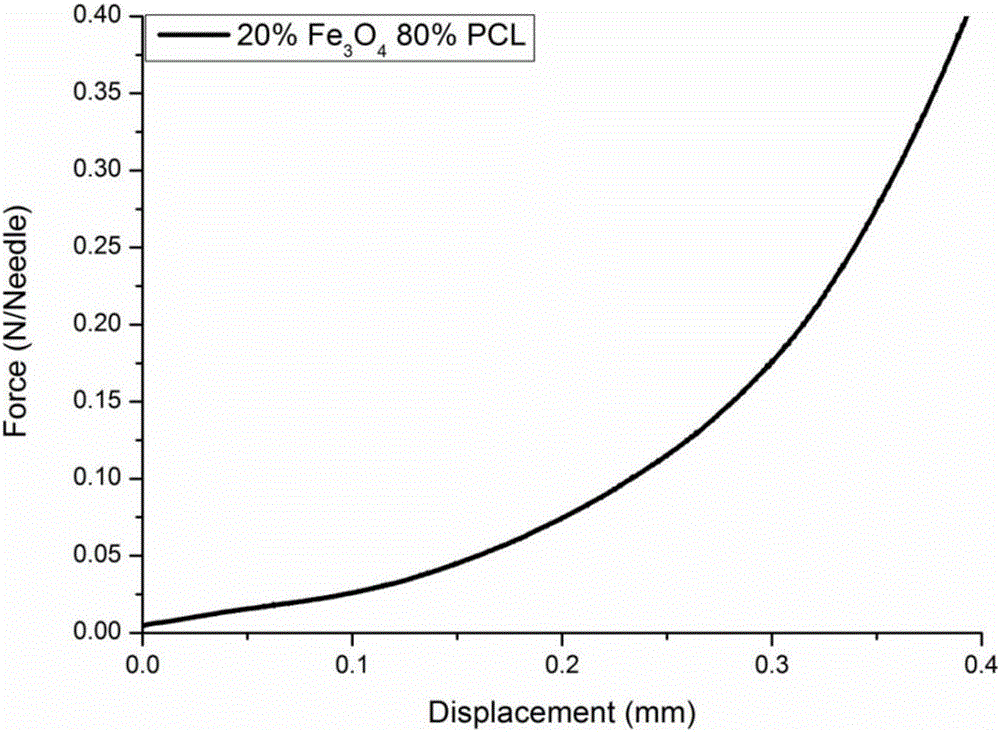
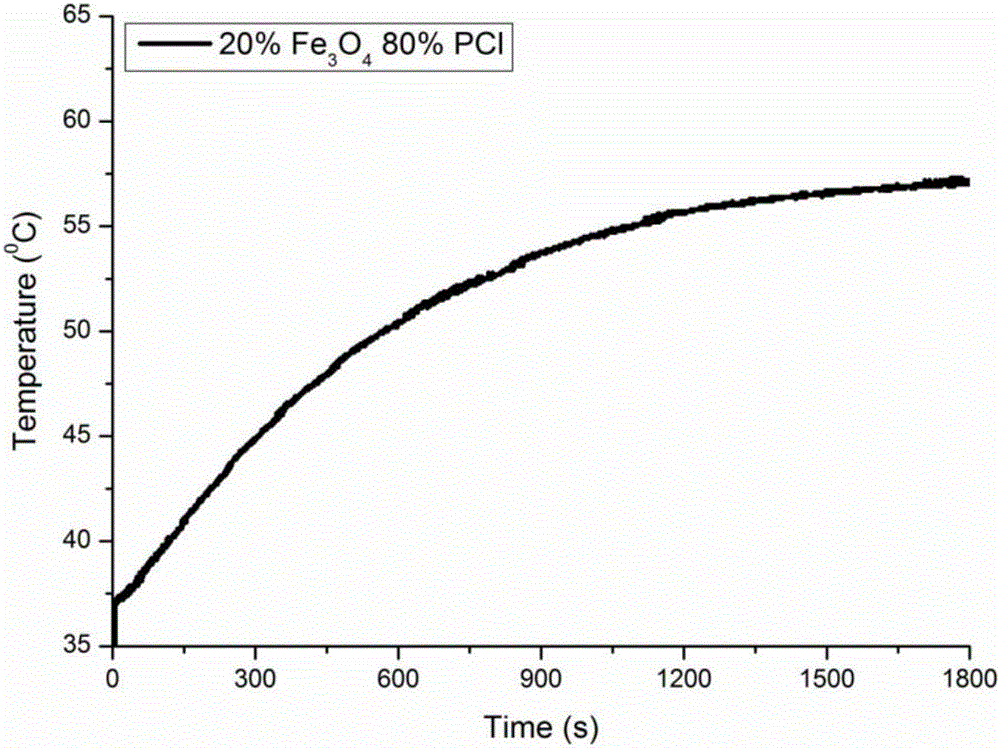
[0032] The drug controlled release transdermal microneedle system provided in this embodiment includes Fe embedded in the PCL. 3 o 4 Nanoparticles and the Mimetic Drug Rhodamine B. The preparation method of the drug controlled release transdermal microneedle system comprises the following steps:
[0033] Step 1, prepare the slurry: take 0.4g of PCL (polycaprolactone, a biopolymer material) and add it to an appropriate amount of chloroform, and seal it at room temperature for 4 hours to completely dissolve it in chloroform, and add 0.1g of Fe to the above solution 3 o 4 nanoparticles, stirred in a fume hood for 1-2h to make Fe 3 o 4 The nanoparticles are fully dispersed in the chloroform solution, and the solution is adjusted to an appropriate viscosity (5000~8000mPa.s).
[0034]Step 2, centrifugal casting molding: add 0.2mL of the slurry in step 1 to the PDMS mold, centrifuge in a centrifuge at 4000rpm for 15min, take it out, then add 0.2mL of the slurry to the mold again...
Embodiment 2
[0044] The drug controlled release transdermal microneedle system provided in this example is a drug controlled release transdermal microneedle system added with a low melting point monomer TMC to adjust the release performance on the basis of Example 1, and its preparation method is as follows:
[0045] Step 1, configure the slurry: take an appropriate amount of 0.3g PCL and add it to an appropriate amount of chloroform, and leave it sealed at room temperature for 4 hours to completely dissolve in chloroform, add 0.1g of TMC (trimethylene carbonate) and 0.1 g of Fe 3 o 4 Nanoparticles and drug Rhodamine B (2.5mg), stirred in a fume hood for 1-2h to make TMC and Fe 3 o 4 The nanoparticles are fully dispersed in the chloroform solution, and the solution is adjusted to an appropriate viscosity (5000~8000mPa.s);
[0046] Step 2, centrifugal casting molding: add 0.2mL of the slurry in step 1 to the PDMS mold, centrifuge in a centrifuge at 4000rpm for 15min, take it out; then ad...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Height | aaaaa | aaaaa |
| Bottom width | aaaaa | aaaaa |
| Height | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com