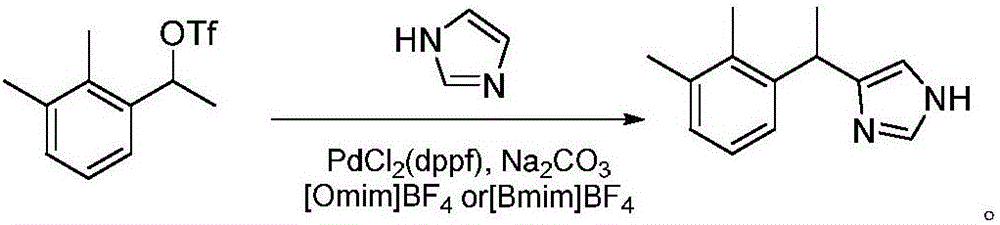Method for synthesizing dexmedetomidine hydrochloride intermediate
A technology for dexmedetomidine hydrochloride and intermediates, applied in the field of pharmaceutical chemical synthesis, can solve the problems of chiral product dependence on resolution, unsatisfactory synthesis yield, and short reaction time, achieving short reaction time and high selectivity , the effect of improving the utilization rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
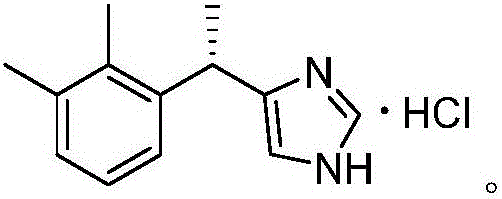
[0029] Synthesis of Dexmedetomidine Hydrochloride Intermediate (4-[1-(2,3-Dimethylphenyl)ethyl]-1H-imidazole)
[0030] In the three-necked flask, in the presence of nitrogen, 31.8 g (300 mmol) of sodium carbonate, 28.2 g (100 mmol) of 1-(1-trifluoromethanesulfonate) ethyl-2,3-dimethylbenzene, [1, 1'-bis(diphenylphosphino)ferrocene]palladium dichloride 3.5g (5mmol), imidazole 8.2g (120mmol) and 52ml[BuPy]BF 4 , stirred and reacted at 70°C for 30 minutes, after monitoring the reaction, poured into water, extracted with dichloromethane, washed the organic phase three times with water, dried the organic phase with anhydrous sodium sulfate, concentrated under reduced pressure, and recrystallized petroleum ether to obtain 4-[1-( 2,3-Dimethylphenyl)ethyl]-1H-imidazole 17.6g, yield 84.7%, (S)-4-[1-(2,3-Dimethylphenyl)ethyl]-1H -Imidazole ee value 79.89%.
[0031] 1 HNMR (400MHz, CDCl 3 ): δ7.35(s,1H),7.08-6.95(m,3H),6.71(s,1H),4.40(q,J=21.2,7.2,1H),2.29(s,3H),2.31(s ,3H), 1.57 (d...
Embodiment 2
[0033] Synthesis of Dexmedetomidine Hydrochloride Intermediate (4-[1-(2,3-Dimethylphenyl)ethyl]-1H-imidazole)
[0034] In the three-necked flask, in the presence of nitrogen, 21.2 g (200 mmol) of sodium carbonate, 28.2 g (100 mmol) of 1-(1-trifluoromethanesulfonate) ethyl-2,3-dimethylbenzene, [1, 1'-bis(diphenylphosphino)ferrocene]palladium dichloride 10g (15mmol), imidazole 8.9g (130mmol) and 52ml[BuPy]BF 4 , stirred and reacted at 80° C. for 30 minutes, after monitoring the reaction, poured into water, extracted with dichloromethane, washed the organic phase three times with water, dried the organic phase with anhydrous sodium sulfate, concentrated under reduced pressure, and recrystallized petroleum ether to obtain 4-[1-( 2,3-Dimethylphenyl)ethyl]-1H-imidazole 17.1g, yield 82.2%, (S)-4-[1-(2,3-Dimethylphenyl)ethyl]-1H -Imidazole ee value 81.02%.
Embodiment 3
[0036] Synthesis of Dexmedetomidine Hydrochloride Intermediate (4-[1-(2,3-Dimethylphenyl)ethyl]-1H-imidazole)
[0037] In a three-necked flask, in the presence of nitrogen, add 40 g of sodium carbonate, 28.2 g (100 mmol) of 1-(1-trifluoromethanesulfonate) ethyl-2,3-dimethylbenzene, [1,1'-bis (Diphenylphosphino) ferrocene] palladium dichloride 7g (10mmol), imidazole 10.2g (150mmol) and 52ml [BuPy] BF4 , stirred and reacted at 90°C for 30 minutes, and after monitoring the reaction, poured into water, extracted with dichloromethane, washed the organic phase three times with water, dried the organic phase with anhydrous sodium sulfate, concentrated under reduced pressure, and recrystallized petroleum ether to obtain 4-[1-( 2,3-Dimethylphenyl)ethyl]-1H-imidazole 17.2g, yield 82.5%, (S)-4-[1-(2,3-Dimethylphenyl)ethyl]-1H -Imidazole ee value 82.41%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com