3, 4-dihydropyrimidine-2(1H)-ketone and derivatives thereof and synthesis method and application thereof
A technology of dihydropyrimidine and synthesis method, which is applied in chemical instruments and methods, luminescent materials, organic chemistry, etc., to achieve the effects of simple reaction method, few by-products and stable performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
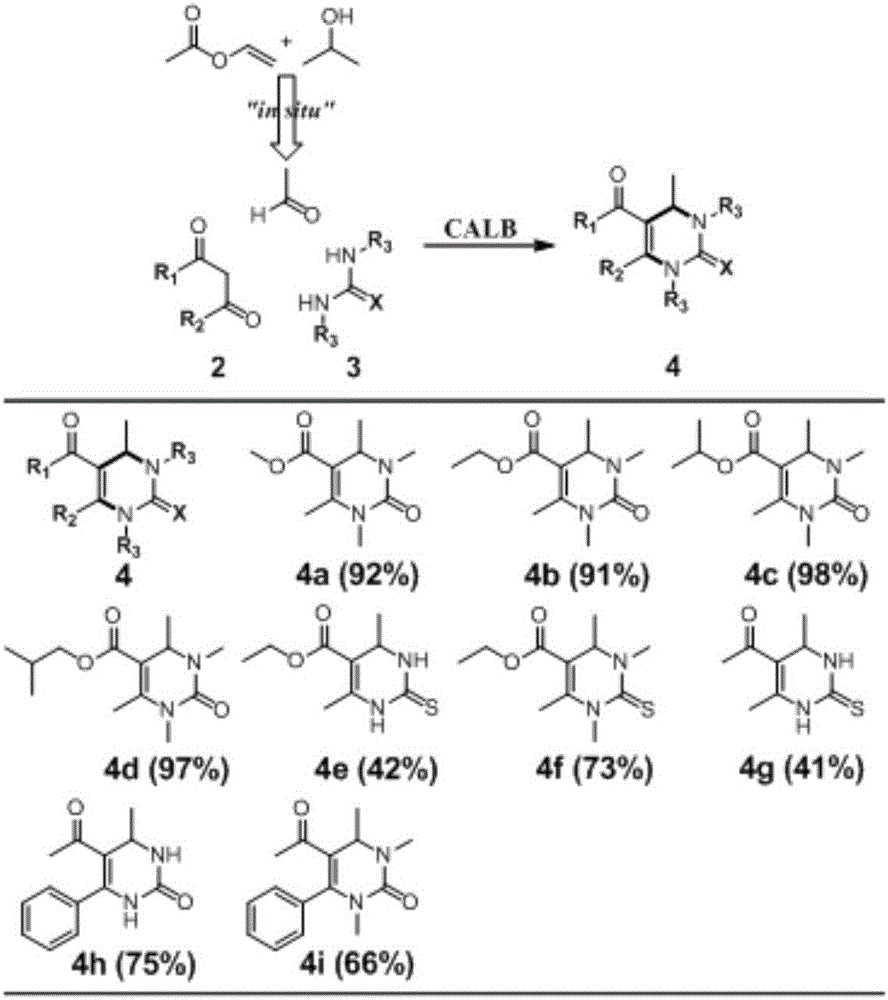
[0024] A synthetic method for 3,4-dihydropyrimidin-2(1H)-one and derivatives thereof, comprising:
[0025] Add 0.2mmol of urea, 0.6mmol of β-dicarbonyl compound, 10mg of CAL-B, 0.2mL of vinyl acetate, 0.2mL of isopropanol and 0.16mL of deionized water into a 10mL round bottom flask;
[0026] The above materials were placed in a constant temperature shaker at 60°C and a rotating speed of 200rpm to react for 2 days. After the reaction, the target product was separated by column chromatography (silica gel: 200-300 mesh, mobile phase: petroleum ether / ethyl acetate). dried in vacuum to obtain;
[0027] Its reaction formula is:
[0028]
[0029] In the above reactants, when R in the β-dicarbonyl compound and urea adopts different groups, the above nine derivatives of 4a, 4b, 4c, 4d, 4e, 4f, 4g, 4h and 4i can be generated and the yield when the above-mentioned derivatives are generated separately;
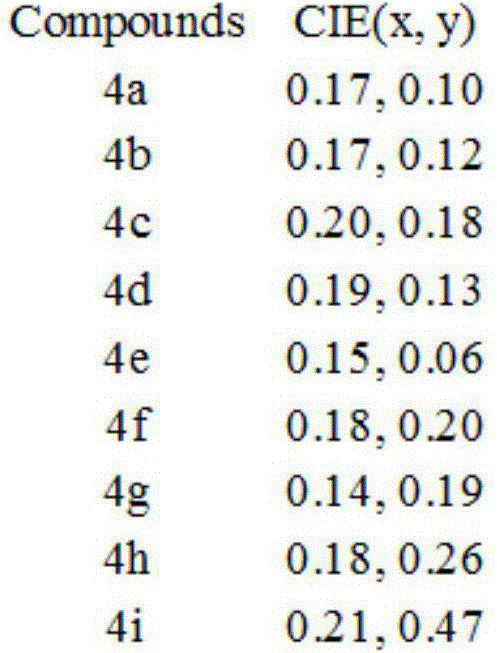
[0030] The fluorescence spectra of the above nine compounds in the solid state ...
Embodiment 2
[0034] A synthetic method for 3,4-dihydropyrimidin-2(1H)-one and derivatives thereof, comprising:
[0035] Add 0.2mmol of urea, 0.4mmol of β-dicarbonyl compound, 6mg of CAL-B, 0.17mL of vinyl acetate, 0.15mL of isopropanol and 0.10mL of deionized water into a 10mL round bottom flask;
[0036] The above materials were placed in a constant temperature shaker at 37° C. with a rotation speed of 100 rpm for 3 days. After the reaction, the target product was separated by column chromatography (silica gel: 200-300 mesh, mobile phase: petroleum ether / ethyl acetate). dried in vacuum to obtain;
Embodiment 3
[0038] A synthetic method for 3,4-dihydropyrimidin-2(1H)-one and derivatives thereof, comprising:
[0039] Add 0.2mmol of urea, 0.3mmol of β-dicarbonyl compound, 12mg of CAL-B, 0.19mL of vinyl acetate, 0.18mL of isopropanol and 0.16mL of deionized water into a 10mL round bottom flask;
[0040] The above materials were placed in a constant temperature shaker at 50°C and a rotating speed of 300rpm to react for 1d. After the reaction, the target product was separated by column chromatography (silica gel: 200-300 mesh, mobile phase: petroleum ether / ethyl acetate). dried in vacuum to obtain;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com