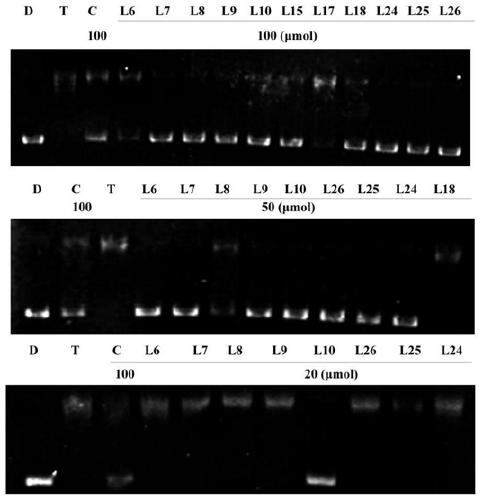
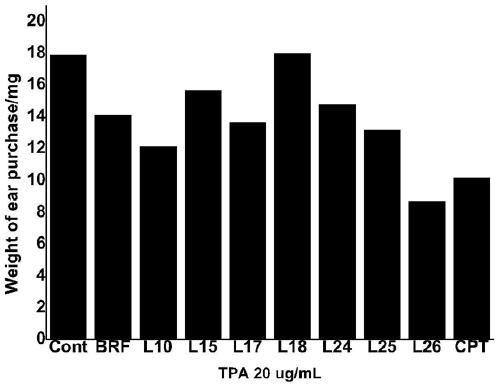
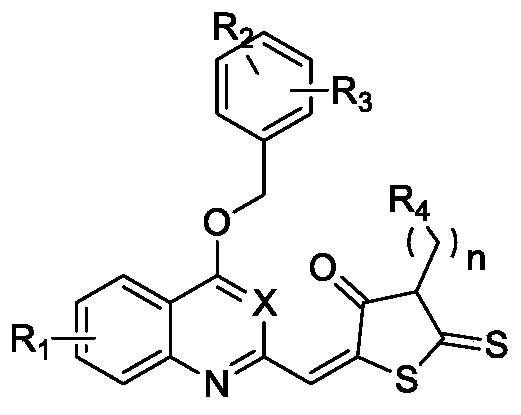
Preparation of a quinoline derivative and its application in anti-inflammation
A technology of derivatives and quinolines, applied in the field of preparation and application of quinoline derivatives, to achieve good correlation, good inhibitory activity, and strong inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: Synthesis of compound A2, B2, C2, D2, E2, F2, G2
[0035] 1:1.5 Aniline and ethyl acetoacetate are catalyzed by polyphosphoric acid and reacted at 130°C for 2h. After the reaction, it was cooled to room temperature, crushed ice was added, and the pH was adjusted to 8 with sodium hydroxide. The solid was precipitated, filtered with suction, dried by infrared, and put directly into the next step without purification.
[0036]
Embodiment 2
[0037] Example 2: Synthesis of Compound A3-A17
[0038]
[0039] 1:1 equivalent of benzyl bromide and A2 solution DMF, add 5 equivalents of potassium carbonate, and react at room temperature for 8 hours. After the reaction, it was extracted with ethyl acetate and water, the organic phase was collected, and purified by silica gel column to obtain a white solid with a yield of 87-93%.
Embodiment 3
[0040] Example 3: Synthesis of Compound A18-A32
[0041]
[0042] Dioxane was used as a solvent, and A3-A17 of 1:1.5 equivalent was reacted with selenium dioxide at 65° C. for 2 hours. After the reaction, it was filtered, the filtrate was collected, and purified by a silica gel column. The yield was 71-85%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com