Preparation method of high-sensitivity mirtazapine molecularly-imprinted electrochemical sensor
A molecular imprinting and high-sensitivity technology, applied in scientific instruments, instruments, analytical materials, etc., can solve problems such as low sensitivity, inappropriate matching of functional monomers, template molecules and cross-linking agents, poor cross-linking rigidity, etc., to achieve Simple equipment, excellent sensitivity, and easy-to-make effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
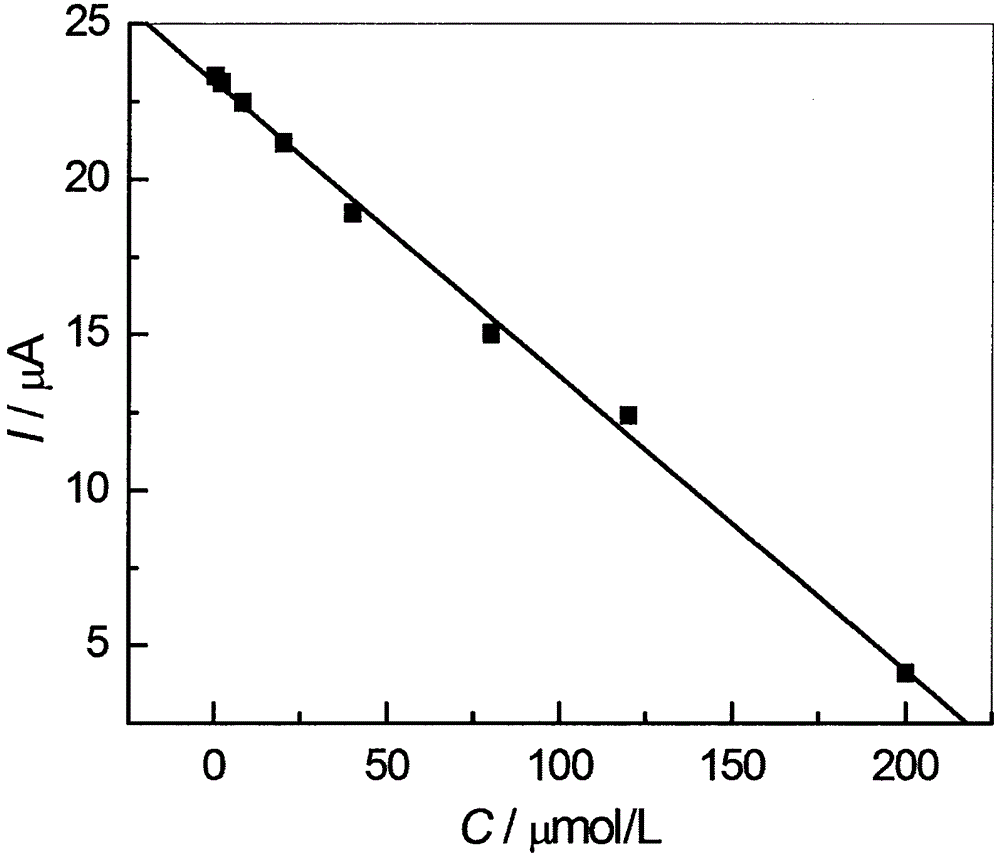
Image
Examples
Embodiment 1
[0015] 1. Treatment of glassy carbon electrodes
[0016] Polish the glassy carbon electrode on a polishing cloth with 1.0 μm, 0.3 μm and 0.05 μm alumina powder in sequence, then put it into nitric acid with a volume ratio of 1:1 for 6 minutes, then put it into absolute ethanol for 6 minutes, and finally Ultrasonic cleaning with pure water.
[0017] 2. Preparation of Molecularly Imprinted Electrochemical Sensor for Mirtazapine
[0018] use water as a solvent to prepare 10ml of nickel sulfate aqueous solution with a concentration of 0.5mol / L, and use N, N-dimethylformamide as a solvent to prepare 50ml of a salicylic acid solution with a concentration of 0.05mol / L, and then take the prepared Add 10ml of nickel sulfate aqueous solution dropwise to the prepared 50ml of salicylic acid solution at a rate of 5 drops / second, stir vigorously at a speed of 3000 rpm during the dropwise addition, and then react in a 100ml hot water kettle at 118°C 11 hours, after cooling, the resulting ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com