Inflammatory myopathy SAE1 self antibody non-radioactive detection method and application thereof
An autoantibody and detection method technology, applied in the biological field, can solve the problems of high cost and high false positive rate of ELISA kits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 Non-radiolabeled immunoprecipitation detection method for SAE1 autoantibodies in inflammatory myopathy
[0029] 1. Materials
[0030] pENTER-SAE1 plasmid (Virgin Bio, CH838665)
[0031] Flag antibody (Sigma-Aldrich, F1804)
[0032] Protein G Protein G magnetic beads (Millipore, LSKMAGG10)
[0033] 2. Method
[0034] A non-radiolabeled immunoprecipitation detection method for SAE1 autoantibodies in inflammatory myopathy, comprising the following steps:
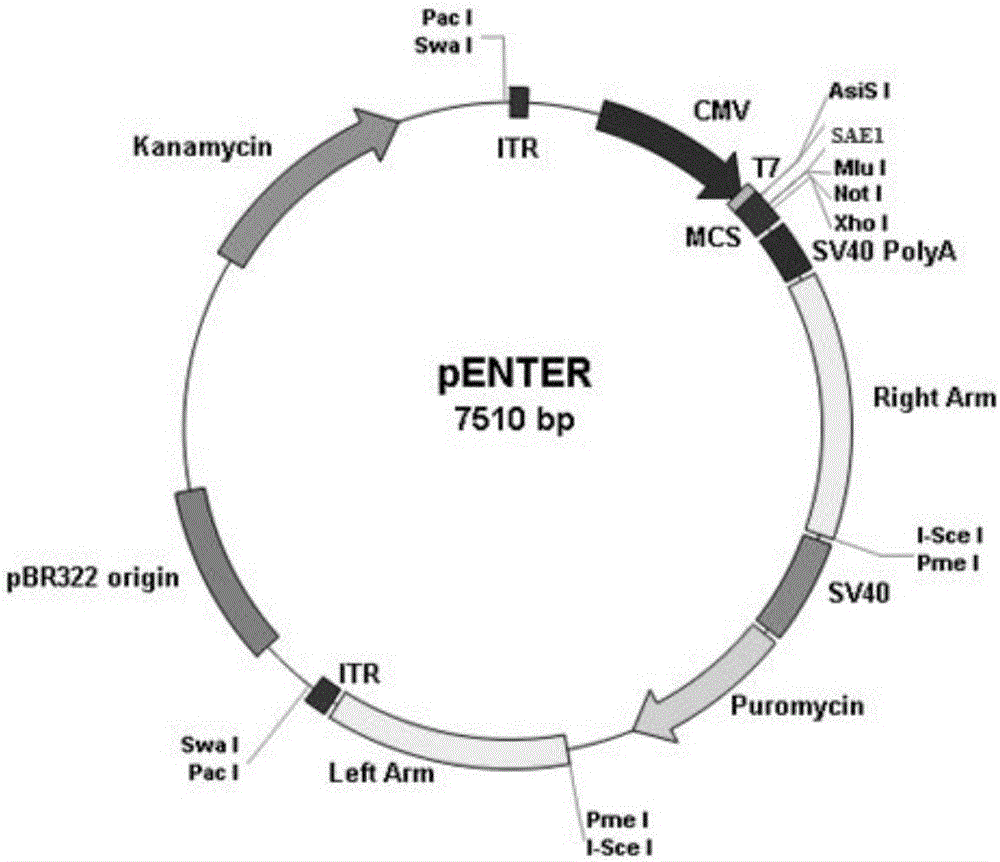
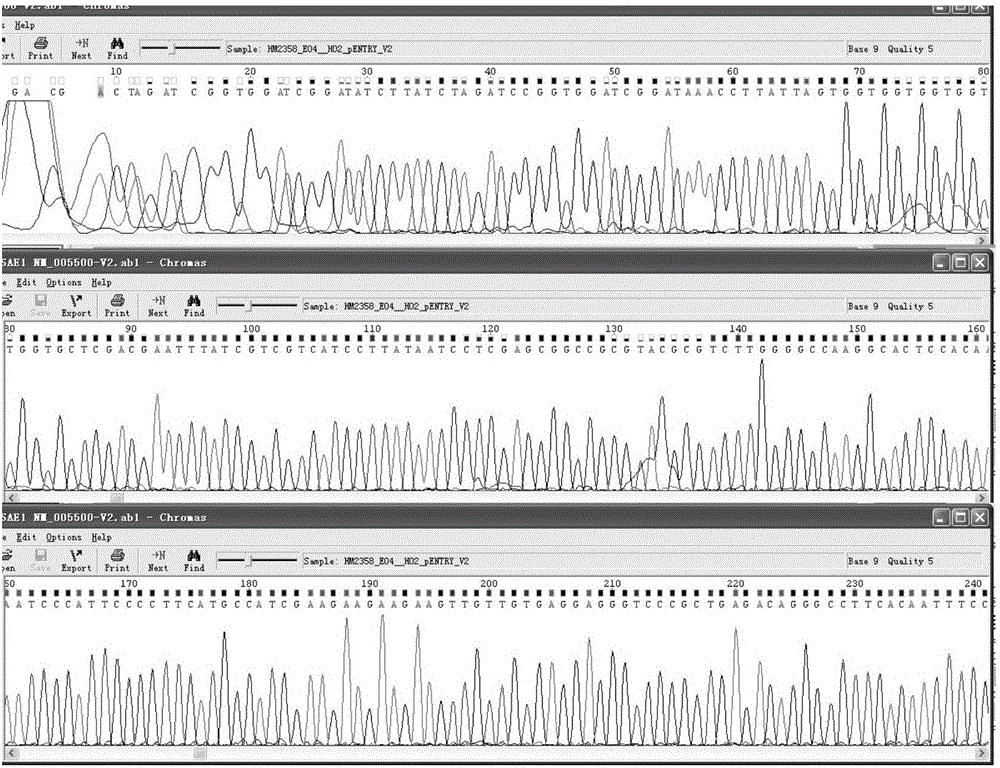
[0035] (1) The pENTER-SAE1 plasmid with the Flag tag (as attached figure 1 shown) for sequencing to confirm the accuracy of the SAE1 sequence and reading frame (as attached figure 2 shown);
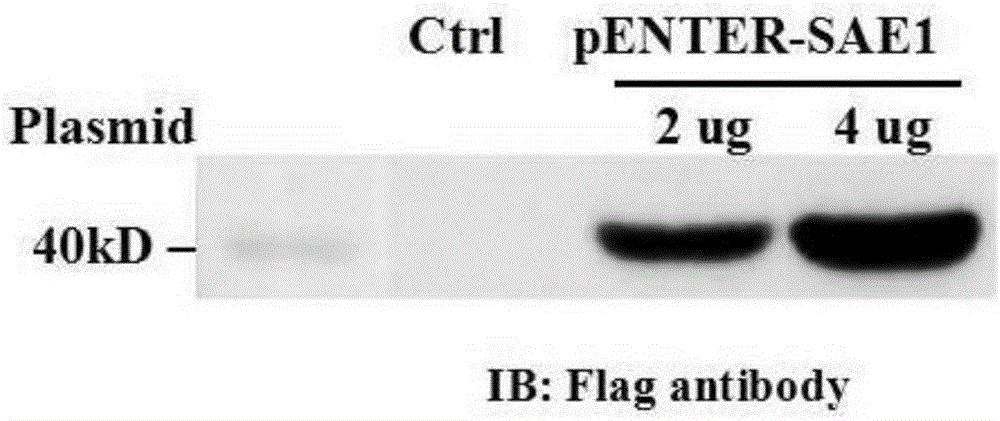
[0036] (2) 2ug pENTER-SAE1 and 4ug pENTER-SAE1 were transiently transfected into human embryonic kidney (HEK) 293 cells for 48 hours, and the HEK293 cells were lysed with SDS lysate, and then SDS-PAGE gel electrophoresis and membrane transfer were performed using Flag antibody. After incubation, it was found that t...
Embodiment 2
[0038] Example 2 Western blot detection method for inflammatory myopathy SAE1 autoantibody
[0039] 1. Materials
[0040] pENTER-SAE1 plasmid (Virgin Bio, CH838665)
[0041] Flag antibody (Sigma-Aldrich, F1804)
[0042] Protein G Protein G magnetic beads (Millipore, LSKMAGG10)
[0043] SAE1 antibody positive serum (1:100 dilution)
[0044] Rabbit anti-human IgG H&L (1:5000, Abcam, ab6759))
[0045] 2. Method
[0046] The immunoblotting method for detecting SAE1 autoantibodies in inflammatory myopathy comprises the following steps:
[0047] (1) 2ug pENTER-SAE1 and 4ug pENTER-SAE1 were transiently transfected into HEK293 cells for 48 hours respectively, and the HEK293 cells were lysed with SDS lysate, then subjected to SDS-PAGE gel electrophoresis and membrane transfer, and incubated with Flag antibody, and it was found that transfected pENTER - There is a specific band of about 40kDa in the HEK293 cells of the SAE1 plasmid (as attached image 3 shown), the above results ...
Embodiment 3
[0049] Example 3 detects the positive rate of SAE1 antibody in the serum of 52 patients with inflammatory myopathy
[0050] The positive rate of SAE1 antibody in the serum of patients with inflammatory myopathy includes the following steps:
[0051] (1) Inoculate 30% HEK293 cells on 75cm 2 Cell culture dish, cultivated at 37°C for less than 24 hours;
[0052] (2) Transfect HEK293 cells with 20ug pENTER-SAE1 plasmid for 48 hours;
[0053] (3) HEK293 cells were lysed with 1 ml of RIPA lysate containing protease inhibitors, and the protein was quantified;
[0054] (4) Using Flag antibody to confirm the overexpression of SAE1 in HEK293 cells;
[0055] (5) Take 20ul of the test serum, positive serum, and negative serum from patients with inflammatory myopathy to perform immunoprecipitation with the cell lysate overexpressing SAE1, perform SDS-PAGE gel electrophoresis, and then detect with Flag antibody to determine the SAE1 antibody The positive rate in patients with inflammato...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com