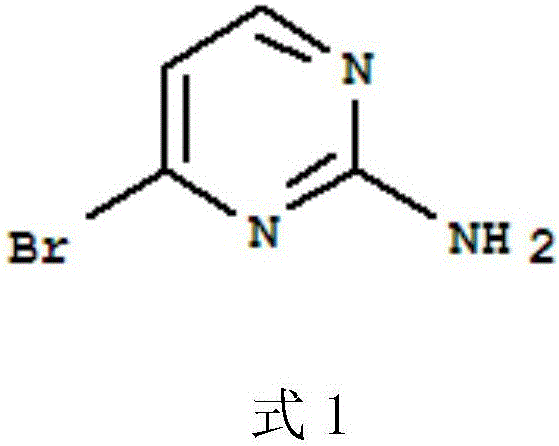
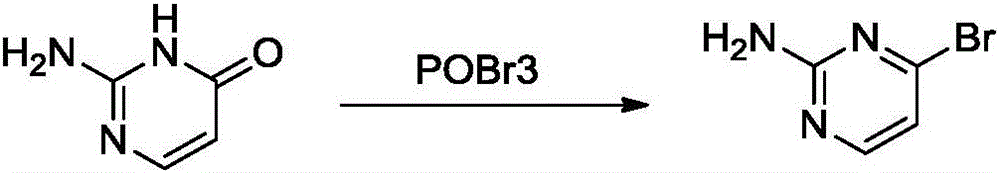
Preparation method of 2-amio-4-bromine pyrimidine
A technology of bromopyrimidine and amino, which is applied in the field of preparation of 2-amino-4-bromopyrimidine, can solve the problems of high raw material cost, low reaction yield, poor economic benefit and environmental impact, and reduce raw material cost and The effect of production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] The first step: the synthesis of 2,4-dibromopyrimidine
[0019] A mixture of phosphorus oxybromide (40.13g, 0.14mol) and uracil (3g, 0.027mol) was stirred at 125°C for 2 hours, the reaction was cooled to room temperature, and the reaction mixture was slowly poured into 500g of ice water, and washed with solid sodium bicarbonate Neutralization reaction. The aqueous phase was extracted twice with 150 ml of dichloromethane. The organic phase was dried and spin-dried under reduced pressure to obtain 6.2 g of crude product, which was purified by flash silica gel column to obtain 5 g of white solid product (yield: 78.5%).
[0020] The second step: the synthesis of 2-amino-4-bromopyrimidine
[0021] 2,4-Dibromopyrimidine (5g, 0.021mol) and ammonia water (28%, 100ml) were stirred at 25°C for 16 hours. After the reaction was completed, the solid was filtered and washed with ether to obtain 3g of crude product. Heating with an oil bath, the crude product was dissolved in metha...
Embodiment 2
[0026] The first step: the synthesis of 2,4-dibromopyrimidine
[0027] A mixture of phosphorus oxybromide (23.22g, 0.081mol) and uracil (3g, 0.027mol) was stirred at 120°C for 2 hours, the reaction was cooled to room temperature, and the reaction mixture was slowly poured into 500g of ice water, and washed with solid sodium bicarbonate Neutralization reaction. The aqueous phase was extracted twice with 150 ml of dichloromethane. The organic phase was dried and spin-dried under reduced pressure to obtain 6 g of crude product, which was purified by column to obtain 4.95 g of white solid product (yield: 77.7%).
[0028] The second step: the synthesis of 2-amino-4-bromopyrimidine
[0029] 2,4-Dibromopyrimidine (5g, 0.021mol) and ammonia in tetrahydrofuran (28%, 100ml) were stirred at 20°C for 16 hours. After the reaction was complete, the solid was filtered and washed with ether to obtain 3g of crude product. Heating with an oil bath, the crude product was dissolved in methanol...
Embodiment 3
[0033] The first step: the synthesis of 2,4-dibromopyrimidine
[0034] A mixture of phosphorus oxybromide (54.18g, 0.189mol) and uracil (3g, 0.027mol) was stirred at 130°C for 2 hours, the reaction was cooled to room temperature, and the reaction mixture was slowly poured into 500g of ice water, and washed with solid sodium bicarbonate Neutralization reaction. The aqueous phase was extracted twice with 150 ml of dichloromethane. The organic phase was dried and spin-dried under reduced pressure to obtain 6.1 g of crude product, which was purified by column to obtain 4.9 g of white solid product (yield: 76.92%).
[0035] The second step: the synthesis of 2-amino-4-bromopyrimidine
[0036] 2,4-Dibromopyrimidine (5g, 0.021mol) and methanolic ammonia solution (28%, 100ml) were stirred at 30°C for 16 hours. After the reaction was complete, the solid was filtered and washed with ether to obtain 3g of crude product. Heating with an oil bath, the crude product was dissolved in metha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com