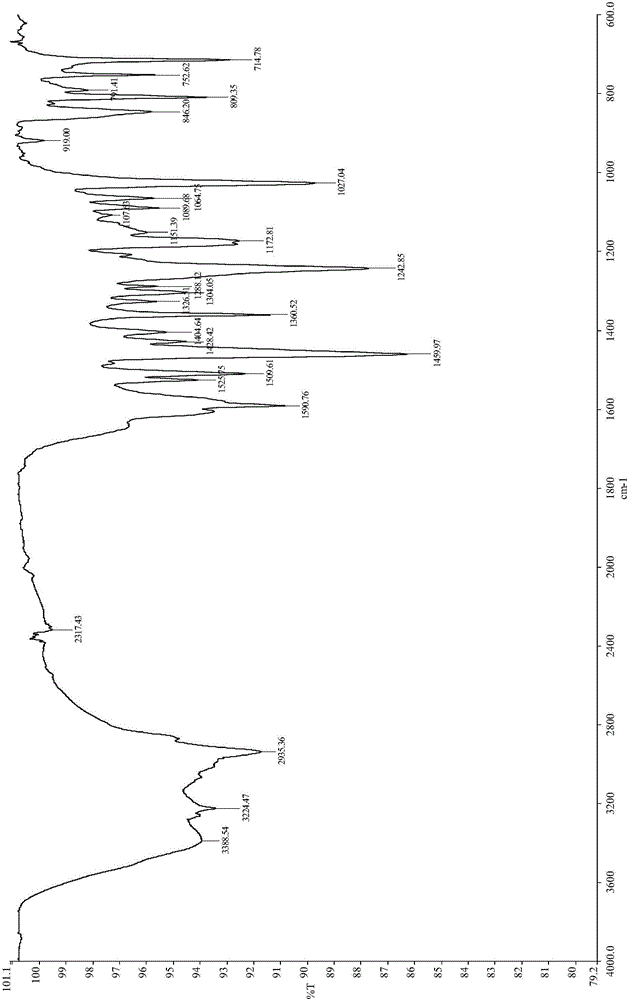
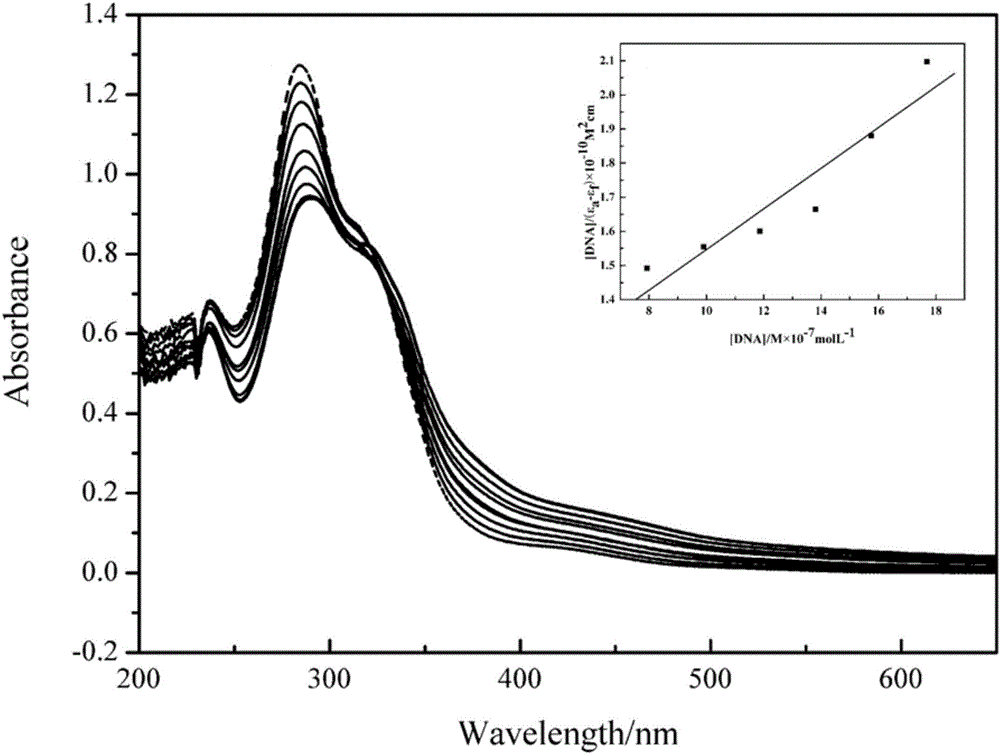
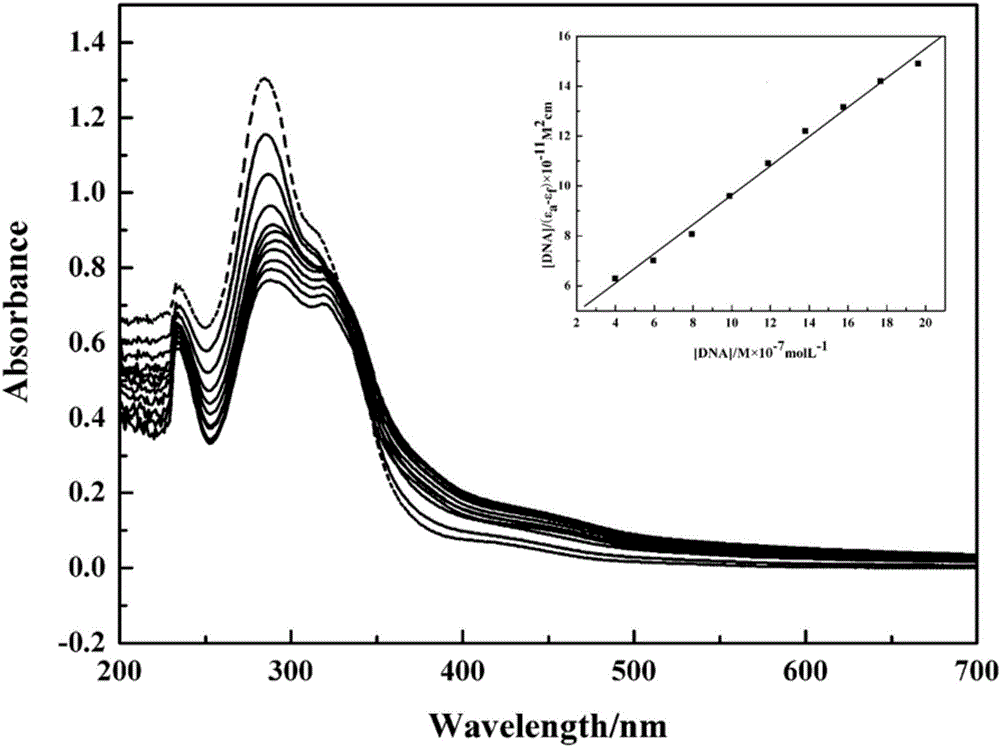
Bladder cancer cell inhibitor and preparation method thereof
A technology of bladder cancer cells and inhibitors, applied in chemical instruments and methods, platinum group organic compounds, organic chemistry, etc., can solve problems such as severe adverse reactions, low immunity, and serious physical damage, and achieve enhanced cell transmembrane ability , stable molecular structure, strong anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] 1. The preparation method of the present invention:
[0043] (1) Preparation of compound 2: Weigh 1 mol of o-phenanthroline-5,6-dione (compound 1) and 1 mol of 4-methoxybenzaldehyde, add 1000 mL of ethanol, and heat to 80°C to make the o-phenanthrene Rholine-5,6-dione was dissolved, then 3850g of ammonium acetate was added in batches, heated to reflux in a water bath at 80℃ for 4h, and cooled to room temperature; under ice bath conditions, stirring, slowly adding concentrated ammonia (25-28%) Until the solution is neutral, a yellow precipitate (compound 2) of 2-[4-(methoxy)-phenyl]imidazo[4,5-f][1,10]-phenanthroline (compound 2) is obtained, and the precipitate is filtered , Ethanol washing, vacuum drying;
[0044] (2) Preparation of complex 3: Prepare potassium chloroplatinate solution in advance, add 0.8 mL of water to 2.4 mmol potassium chloroplatinate, heat to dissolve at 60°C, add 2 mL of dimethyl sulfoxide, heat and stir at 60°C for 5 min That's it.
[0045] Add 400 m...
Embodiment 2
[0067] 1. The preparation method of the present invention:
[0068] (1) Preparation of compound 2: Weigh 2 mol of o-phenanthroline-5,6-dione (compound 1) and 3 mol of 4-methoxybenzaldehyde, and add 2L Ethanol And 4000g of ammonium acetate, Under 80℃ water bath , Heating to reflux for 4h, and cooling to room temperature; under ice bath conditions, stirring, slowly adding 200 mL of concentrated ammonia (25-28%) to neutrality to obtain a yellow precipitate (compound 2);
[0069] (2) Preparation of complex 3: Prepare a potassium chloroplatinate solution in advance, add 1 mL of water to 2.4 mmol potassium chloroplatinate, heat to dissolve at 50°C, add 3 mL of dimethyl sulfoxide, and heat and stir at 50°C for 10 minutes. can.
[0070] Add 9.6mL to 2.4mmol compound 2 Ethanol , water The bath is heated to 85°C and heated for 20 minutes to dissolve compound 2. The pre-prepared potassium chloroplatinite solution is slowly dropped into the compound 2 solution, and a yellow precipitate is qu...
Embodiment 3
[0086] 1. The preparation method of the present invention:
[0087] (1) Preparation of compound 2: Weigh 2 mol of o-phenanthroline-5,6-dione (compound 1) and 2.5 mol of 4-methoxybenzaldehyde, and add 1.5L of Ethanol , And 3900g of ammonium acetate, Under 85℃ water bath , Heated to reflux 4 h, cooling to room temperature; stirring in an ice bath, slowly adding 180 mL of concentrated ammonia (25-28%) to neutrality to obtain a yellow precipitate (compound 2);
[0088] (2) Preparation of complex 3: Prepare potassium chloroplatinate solution in advance, add 5 mL of water to 2.4 mmol potassium chloroplatinate, heat at 55°C to dissolve, add 2.5 mL of dimethyl sulfoxide, heat at 55°C and stir for 8 min That's it.
[0089] Add 10 mL of 2.4 mmol compound 2 Ethanol , water Heat the bath to 80°C to dissolve compound 2, slowly drop the pre-prepared potassium chloroplatinate solution into the compound 2 solution, and quickly precipitate a yellow precipitate, continue to reflux and stir for 1.5...
PUM
| Property | Measurement | Unit |
|---|---|---|
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com