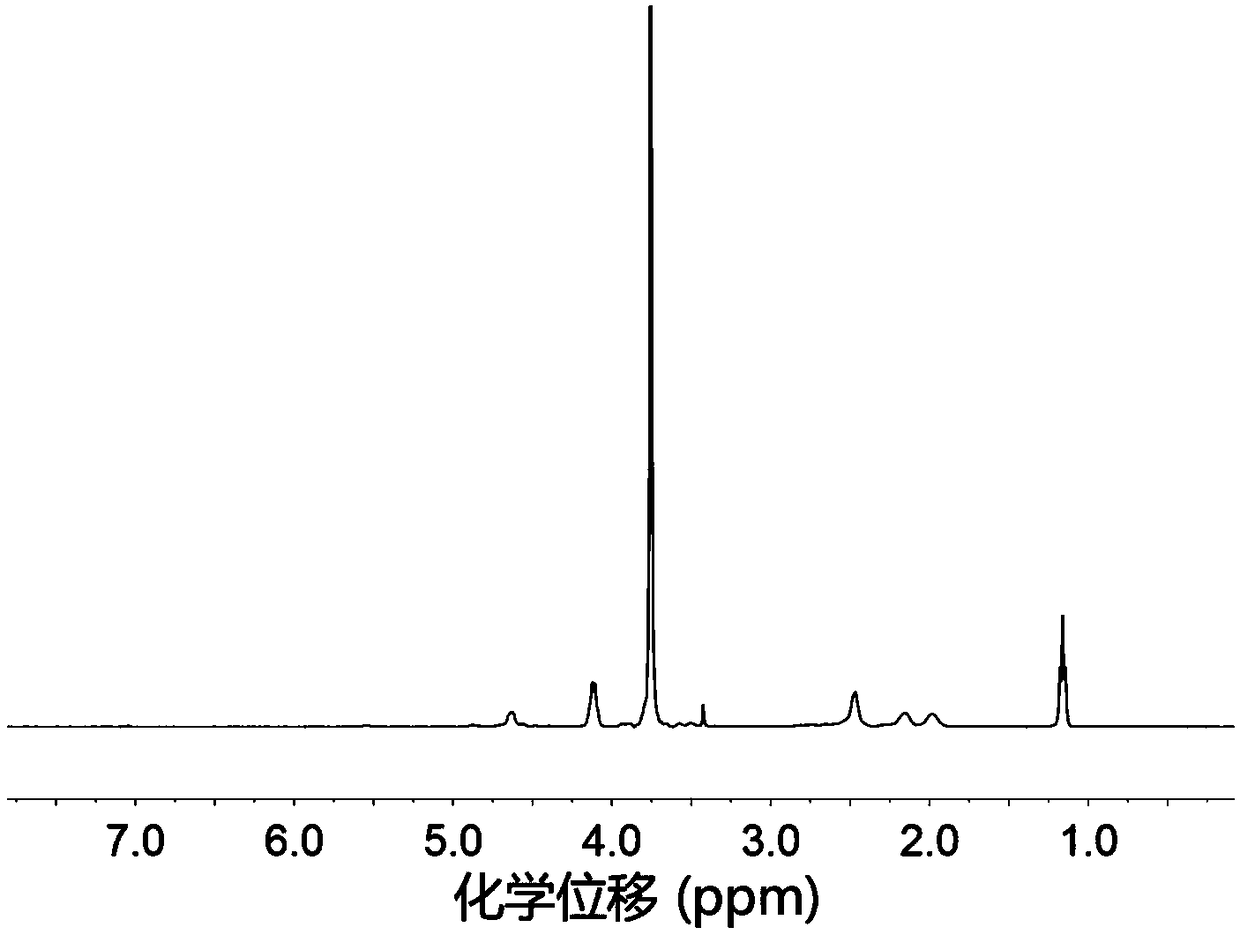
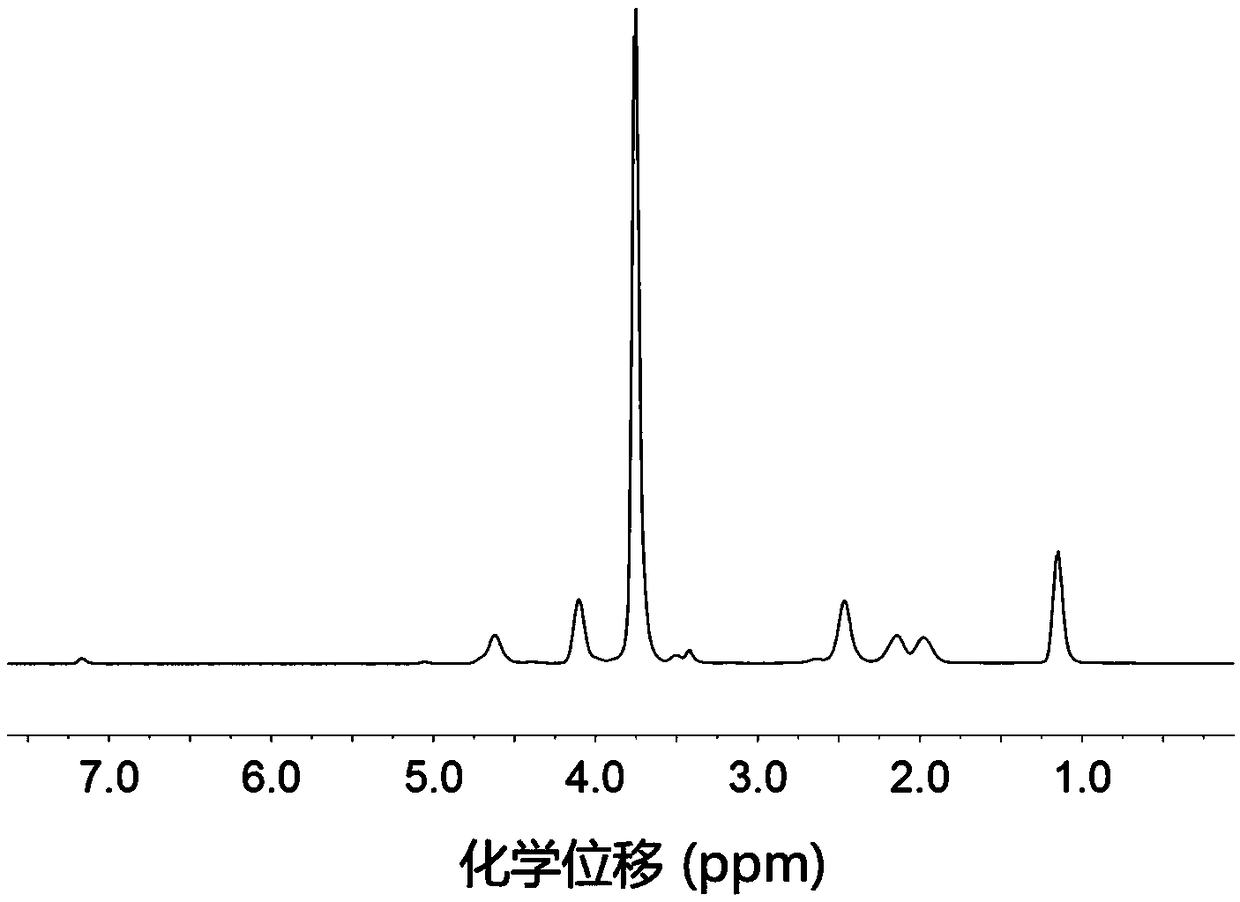
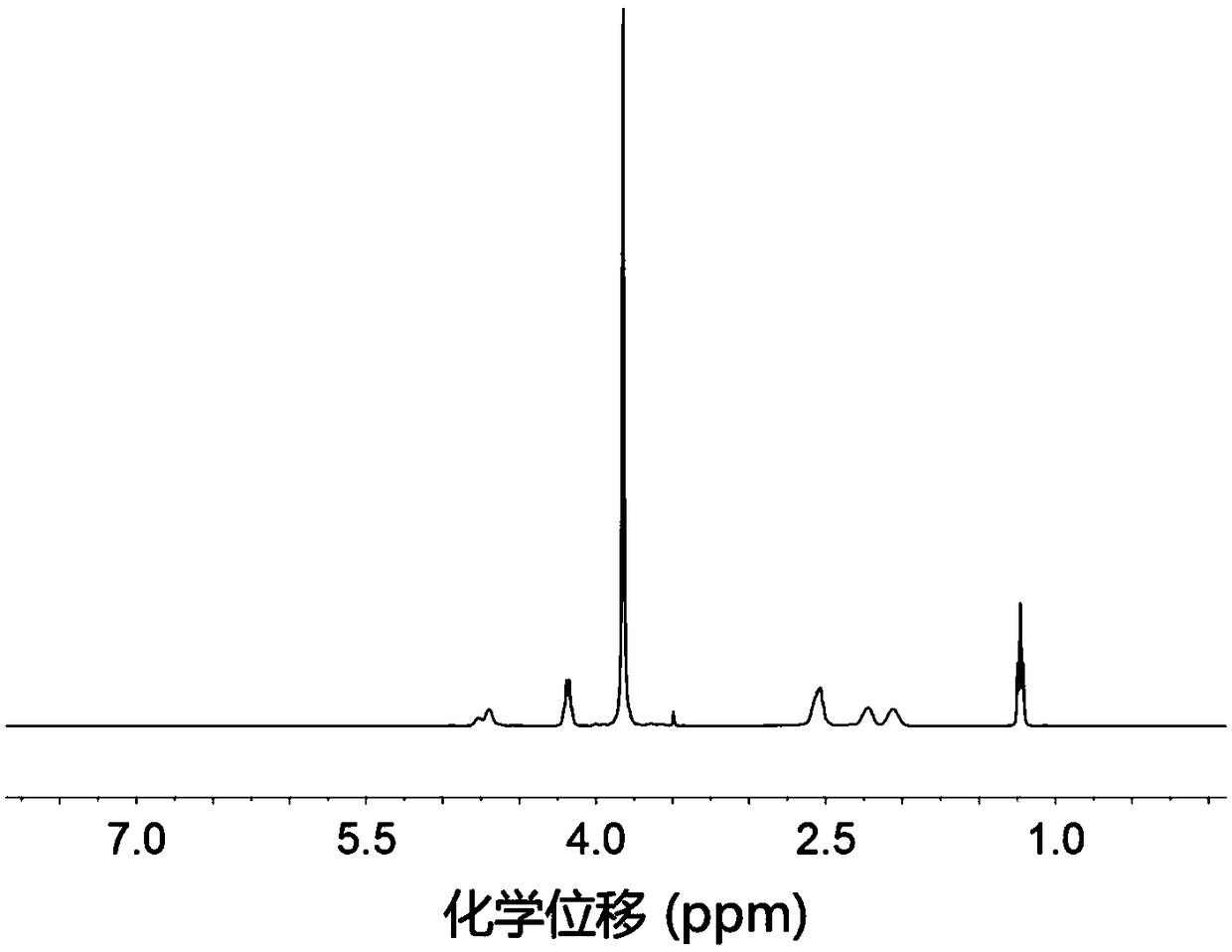
Amino acid block copolymer, its preparation method and temperature-sensitive hydrogel
A block copolymer and hydrogel technology, applied in the field of polyamino acids, can solve the problems of unfavorable temperature-sensitive hydrogel application and single adjustability, and achieve good biodegradability, good adjustability, and good The effect of biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0038] The invention provides a kind of preparation method of block copolymer, comprises the following steps:
[0039] Aminated polyethylene glycol monomethyl ether with a structure of formula IV or aminated polyethylene glycol with a structure of formula V, combined with a structure of formula VI γ-alkyl-L-ethyl glutamate-N- Carboxylic acid internal anhydride and amino acid derivative-N-carboxylic acid internal acid anhydride are polymerized and deprotected to obtain a block polymer;
[0040]
[0041] Among them, m is the degree of polymerization, 5≤m≤227;
[0042] n is the degree of polymerization, 5≤n≤226.
[0043] In the present invention, the aminated polyethylene glycol monomethyl ether having the structure of formula (IV) or the aminated polyethylene glycol having the structure of formula (V) is preferably prepared according to the following method:
[0044] Esterification of polyethylene glycol monomethyl ether or polyethylene glycol with potassium hydroxide and p...
Embodiment 1
[0091] Azeotrope 3.0 g of aminated polyethylene glycol monomethyl ether with a number average molecular weight of 2000 and 100 mL of toluene at 130 ° C for 2 h to remove water, and then drain the remaining toluene under reduced pressure; dissolve the obtained solid in 30 mL In dried N,N-dimethylformamide, the first solution was obtained; 4.52g of γ-ethyl-L-glutamic acid ester-N-carboxylic acid internal anhydride and 0.59g of γ-benzyl-L-glutamine Amino acid ester-N-carboxylic acid internal acid anhydride was dissolved in 60mL of dry N,N-dimethylformamide to obtain a second solution; in a nitrogen atmosphere, the first solution was mixed with the second solution, at room temperature . Stirring and reacting for 72 hours under nitrogen protection; after the reaction, drain N,N-dimethylformamide under reduced pressure, then dissolve the obtained solid in chloroform, settle with ether, filter with suction, and dry to obtain Polyethylene glycol monomethyl ether-polyamino acid ester b...
Embodiment 2
[0094] Azeotrope 3.0 g of aminated polyethylene glycol monomethyl ether with a number average molecular weight of 2000 and 100 mL of toluene at 130 ° C for 2 h to remove water, and then drain the remaining toluene under reduced pressure; dissolve the obtained solid in 30 mL In dried N,N-dimethylformamide, the first solution was obtained; 4.5g of γ-ethyl-L-glutamic acid ester-N-carboxylic acid internal anhydride and 1.18g of γ-benzyl-L-glutamine Amino acid ester-N-carboxylic acid internal acid anhydride was dissolved in 60mL of dry N,N-dimethylformamide to obtain a second solution; in a nitrogen atmosphere, the first solution was mixed with the second solution, at room temperature . Stirring and reacting for 72 hours under nitrogen protection; after the reaction, drain N,N-dimethylformamide under reduced pressure, then dissolve the obtained solid in chloroform, settle with ether, filter with suction, and dry to obtain Polyethylene glycol monomethyl ether-polyamino acid ester bl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com