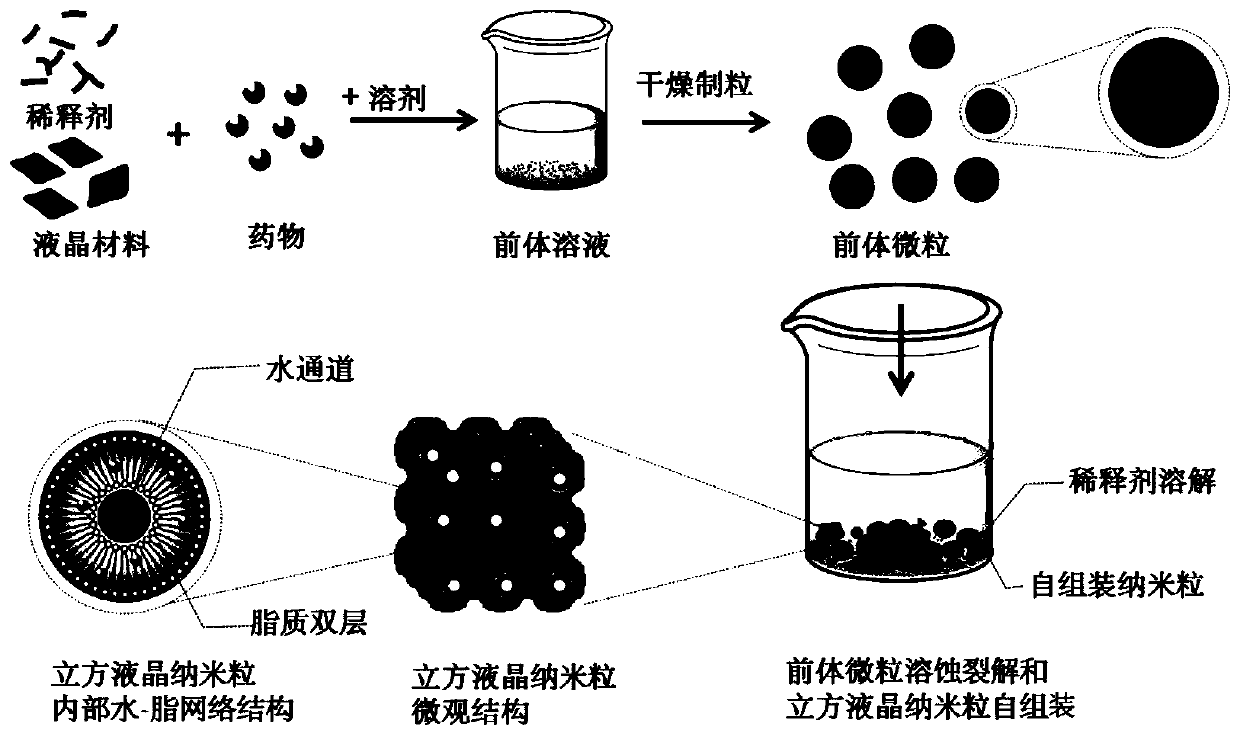
Liquid crystal nanoparticle precursor particles, self-assembled liquid crystal nanoparticles and preparation method thereof
A liquid crystal precursor and nanoparticle technology, which is applied in nanotechnology, nanotechnology, nanomedicine, etc., can solve the problems of liquid crystal nanoparticle drug leakage, unstable dispersion system, and low encapsulation efficiency, achieving good application prospects and improving The effect of complete release degree and high encapsulation rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Example 1: Preparation of Liquid Crystal Nanoparticle Precursor Particles Loaded with Hydroxycamptothecin
[0060] (1) Preparation of liquid crystal precursor solution
[0061] Take by weighing three parts of dimethylacetamide (DMAC), every part of 39.0g, add respectively 0.01g, 0.05g, 0.10g insoluble drug hydroxycamptothecin (the mass fraction of medicine in the obtained liquid crystal nanoparticle precursor microparticle respectively 1%, 5% and 10%), three parts of drug solutions are obtained; in every part of drug solutions, 0.4g of PYT and 0.6g of diluent PVPVA64 (that is, PYT in the obtained liquid crystal nanoparticle precursor particles) are added to melt at 50°C. / PVPVA64 ratio is 4 / 6), vortexed and mixed to obtain three liquid crystal precursor solutions with good fluidity.
[0062] (2) Preparation of liquid crystal nanoparticle precursor particles
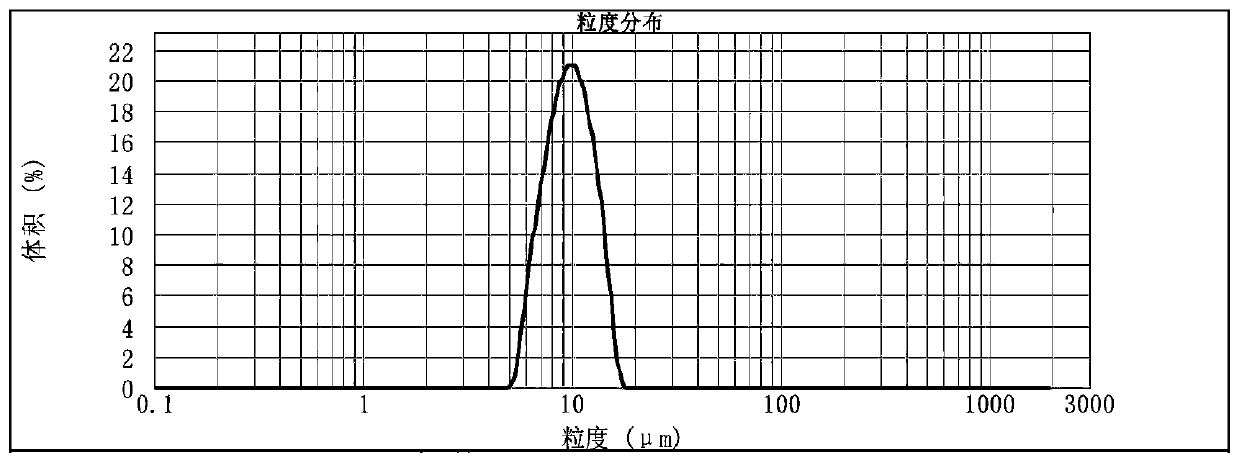
[0063] The uniform liquid crystal precursor solution is solidified and dispersed into liquid crystal nano part...
Embodiment 2
[0075] Example 2: Preparation of Liquid Crystal Nanoparticle Precursor Particles Loaded with Docetaxel
[0076] (1) Preparation of liquid crystal precursor solution
[0077] Weigh three parts of absolute ethanol, each part is 24.0g, add 0.04g insoluble drug docetaxel respectively (that is, the mass fraction of the drug in the obtained liquid crystal nanoparticle precursor particles is respectively 4%) to obtain three parts of drug solutions; GMO and 0.6g, 0.7g and 0.8g of the diluent PVP K30 (that is, the GMO / PVP in the obtained liquid crystal nanoparticle precursor particles) were added respectively in three parts of drug solutions at 47°C of 0.4g, 0.3g and 0.2g. The ratio of K30 is 4 / 6, 3 / 7 and 2 / 8), vortexed and mixed to obtain three liquid crystal precursor solutions with good fluidity.
[0078] (2) Preparation of liquid crystal nanoparticle precursor particles
[0079] The uniform liquid crystal precursor solution is solidified and dispersed into liquid crystal nano par...
Embodiment 3
[0085] Example 3: Preparation of Liquid Crystal Nanoparticles by Dissolution Cleavage and Self-Assembly of Liquid Crystal Nanoparticle Precursor Particles Loaded with Docetaxel
[0086] Weigh 0.01g liquid crystal nanoparticle precursor particles (GMO / PVP / DTX=4 / 6 / 0.04, the preparation method is the same as in Example 2), vortex mix with 0.05g water, when the liquid crystal nanoparticle precursor particles contact with water At the same time, the liquid crystal nanoparticle precursor particles begin to dissolve and crack, and at the same time, the liquid crystal material and water undergo hydration and start to self-assemble to form dispersed liquid crystal nanoparticles.
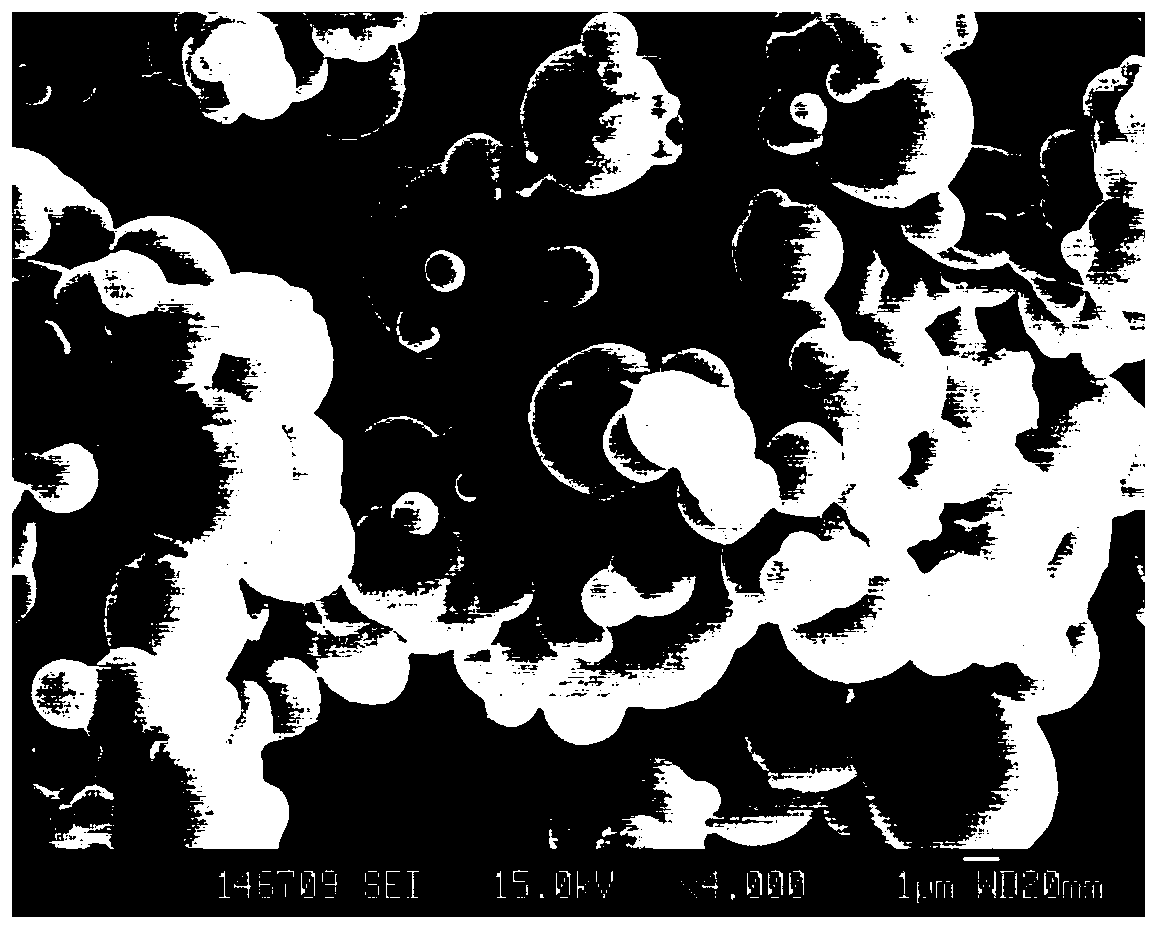
[0087] The new suspension obtained in this example was scanned by a transmission electron microscope to observe the structural changes in the process of liquid crystal nanoparticle precursor particles being dissolved and cracked and self-assembled to obtain liquid crystal nanoparticles. Use a pipette gun to d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com