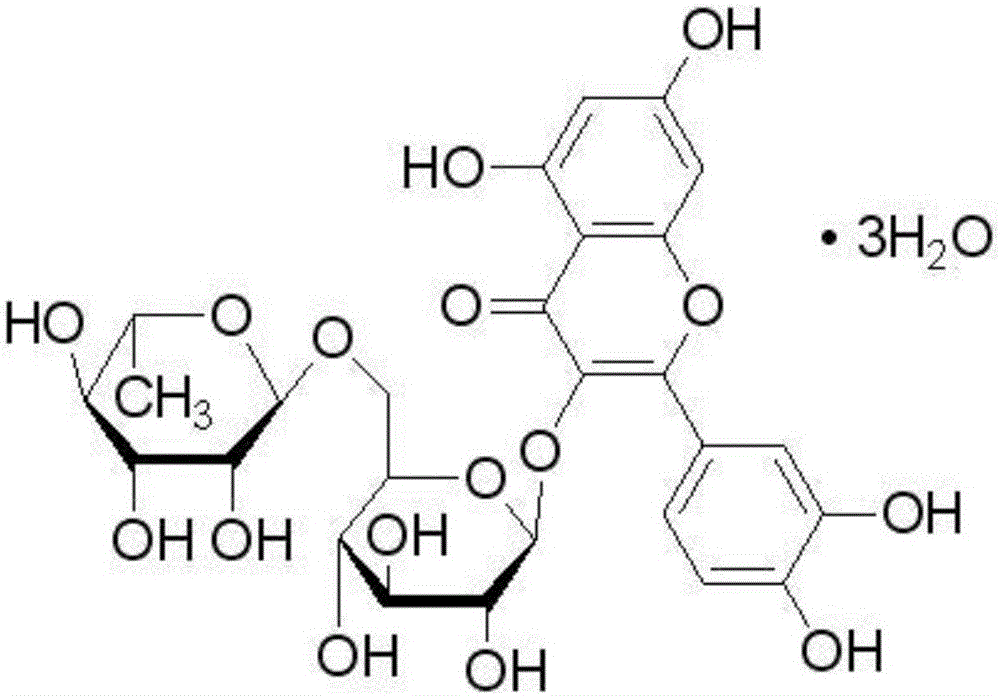
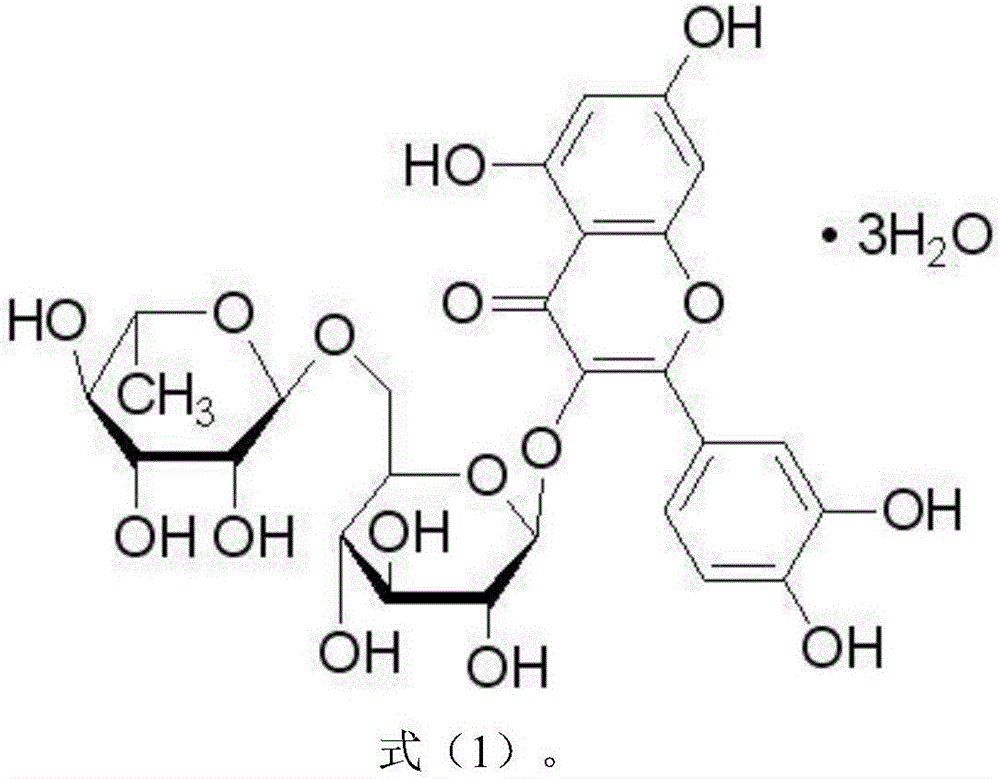
Application of rutin to preparation of drug for promoting mammal immune efficacy
A mammalian immune efficacy technology, applied in drug combinations, medical preparations containing active ingredients, pharmaceutical formulations, etc., can solve problems such as unsatisfactory immune efficacy of vaccines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] 1. Vaccine immunization and administration of rutin
[0029] Group immunization of experimental mice: The experimental BALB / c mice were randomly divided into three groups, A, B, and C, with 10 mice per group. Group A was immunized with a commercial foot-and-mouth disease vaccine and then injected with rutin at a dose of 400 mg / kg. In aqueous solution, group B was immunized with the same vaccine as group A, and group C was a normal saline blank control group with subcutaneous multi-point immunization, 0.2 mL / group. Then the immune cell concentration, cytokine content and antibody level in the serum of the tested mice were detected.
[0030] 2. Detection of antibody concentration
[0031] (1) Antigen coating: Calculate the amount of coating antigen needed according to the number of coating holes, weigh a certain amount of synthetic peptide of viral antigen as coating antigen, dilute it to 5μg / mL with coating solution, and add to 96 wells In the plate, 100μL / well. Afterwards, ...
Embodiment 2
[0067] Take 60 pigs and randomly divide them into 3 groups with 20 pigs in each group, namely the synergistic immunization group, the vaccine group and the blank control group. Among them, the synergistic immunization group was first immunized with a commercial porcine adenovirus vaccine, and then an aqueous solution of rutin was injected at a dose of 400 mg / kg; the vaccine group was only immunized with a commercial porcine adenovirus vaccine; the blank control group was not immunized. After immunization, blood was collected every week to detect antibodies until 7 weeks. As shown in Table 4.
[0068] Table 4 Immunization methods and injection doses of different experimental groups
[0069] Group immunity Immunization method dose Synergistic immune group Vaccine + Rutin Intramuscular injection0.5ml Vaccine group vaccine Intramuscular injection0.5ml Blank control group / / /
[0070] On the 7th day after immunization, the adenovirus group I serotype 4 challenge was performed re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com