Application of yin-nourishing preparation prepared from rhizoma coptidis, radix hedysari, rhizoma curcumae longae, radix rehmanniae and radix scutellariae to preparation of medicine for treating hypertension and/or hyperlipoidemia
A technology for high blood pressure and high blood fat, which is applied in the application field of Wuhuang Yangyin preparation in the preparation of drugs for treating high blood pressure and/or high blood fat, so as to achieve the effect of good blood fat, lower blood fat, and multi-drug selection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1 5
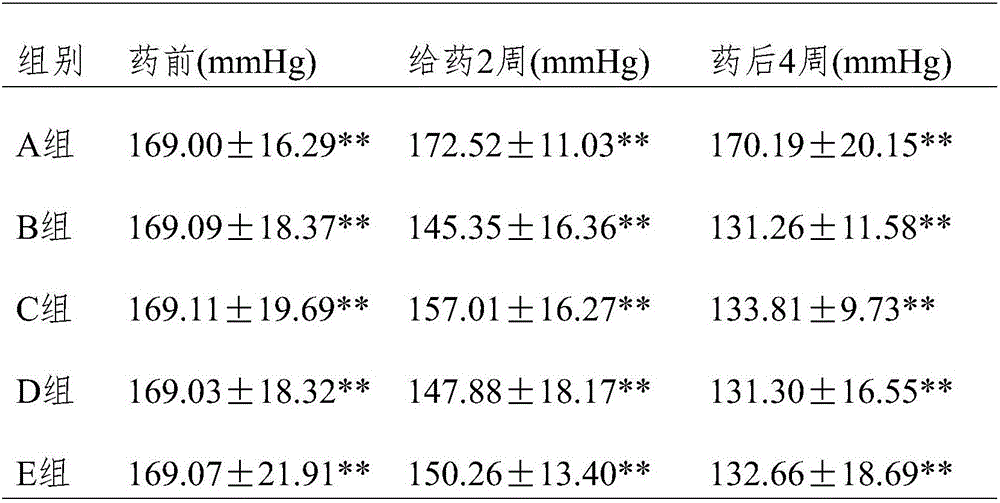
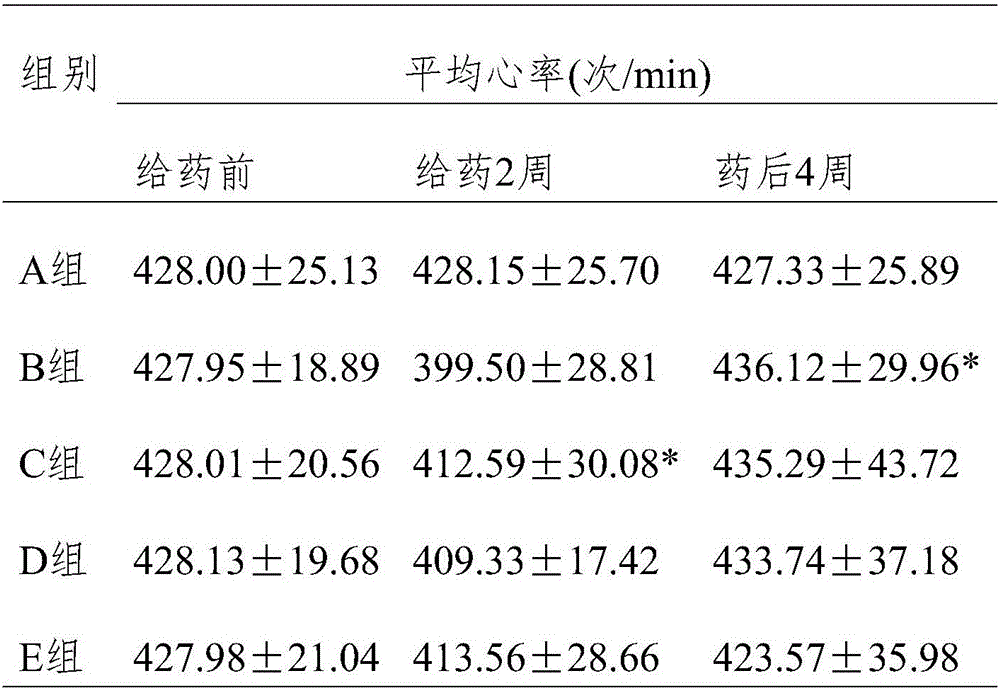
[0017] Experimental Example 1 Effect of Wuhuang Yangyin Preparation on Antihypertensive Efficacy in Rats with Essential Hypertension
[0018] 1 material
[0019] 1.1 Animals
[0020] Adult spontaneously hypertensive rats, male, weighing about 300 g, were provided by Guiyang Medical College.
[0021] 1.2 Experimental drugs
[0022] Irbesartan tablets, produced by Shenzhen Haibin Pharmaceutical Co., Ltd., approval number: Guoyao Zhunzi H20000510;
[0023] Juming Jiangya Pills, produced by Beijing Tongrentang Technology Development Co., Ltd. Pharmaceutical Factory, approval number: Z11020495;
[0024] Wuhuang Yangyin Granules, produced by Chongqing Dongtian Pharmaceutical Co., Ltd., approval number: Z20120014.
[0025] 2 Experimental methods
[0026] 2.1 Grouping and processing:
[0027] Take 50 rats and divide them into 5 groups. Each group will be gavaged / fed once a day for 4 consecutive weeks, specifically: control group (group A): normal saline 2ml / d; irbesartan tablet ...
experiment example 2 5
[0043] Experimental Example 2 Effect of Wuhuang Yangyin Preparation on Blood Lipid Level
[0044] 1 material
[0045] 1.1 Animals
[0046] SD rats, male, weighing about 300 g, were provided by Guiyang Medical College.
[0047] 1.2 Feed
[0048] Cholesterol and sodium cholate were purchased from Jinan Xinniushan Biotechnology Co., Ltd.
[0049] The mass ratio of high-fat feed: 15% lard, 20% sucrose, 2.7% cholesterol, 0.3% sodium cholate, and the balance is basic feed.
[0050] 1.3 Experimental drugs
[0051] Pravastatin sodium tablets, produced by Daiichi Sankyo Pharmaceutical (Shanghai) Co., Ltd., approval number: Guoyao Zhunzi H20060271;
[0052] Qizhi Capsules, produced by Heilongjiang Tiange Pharmaceutical Co., Ltd., approval number: Guoyao Zhunzi B20020452;
[0053] Jiangzhining Granules, produced by Baishan Changbaishan Pharmaceutical Co., Ltd., approval number: Z22023374;
[0054] Each 1 / 3 of Wuhuang Yangyin Granules, Capsules and Tablets was prepared in Examples ...
experiment example 3 5
[0067] Experimental Example 3 Clinical Curative Effect of Wuhuang Yangyin Preparation on Vertigo Patients
[0068] 1. Objects and groups: 20 patients with hypertension and vertigo scale numbers > 4; 15 patients with hypertension complicated with cerebral arterial insufficiency; 10 patients with hypertension complicated with vertebral-basilar artery insufficiency; 25 patients with hypertension complicated with hyperlipidemia; 20 cases of blood lipids; randomly divided into 5 groups, respectively: control group, administration 1-2 group, wherein, no more than 10 cases of the same disease in the control group and administration group;
[0069] 2. Administration and method: administration group 1 was given Zhenju Jiangya Tablets (specification: produced by Changzhou Shenma Pharmaceutical Co., Ltd., approval number: Z20055385), 3 times a day, 1 tablet each time, 0.25g per tablet; the administration group 2 was given Wuhuang Yangyin Granules, once a day, 1 bag each time, 6g per bag;...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com