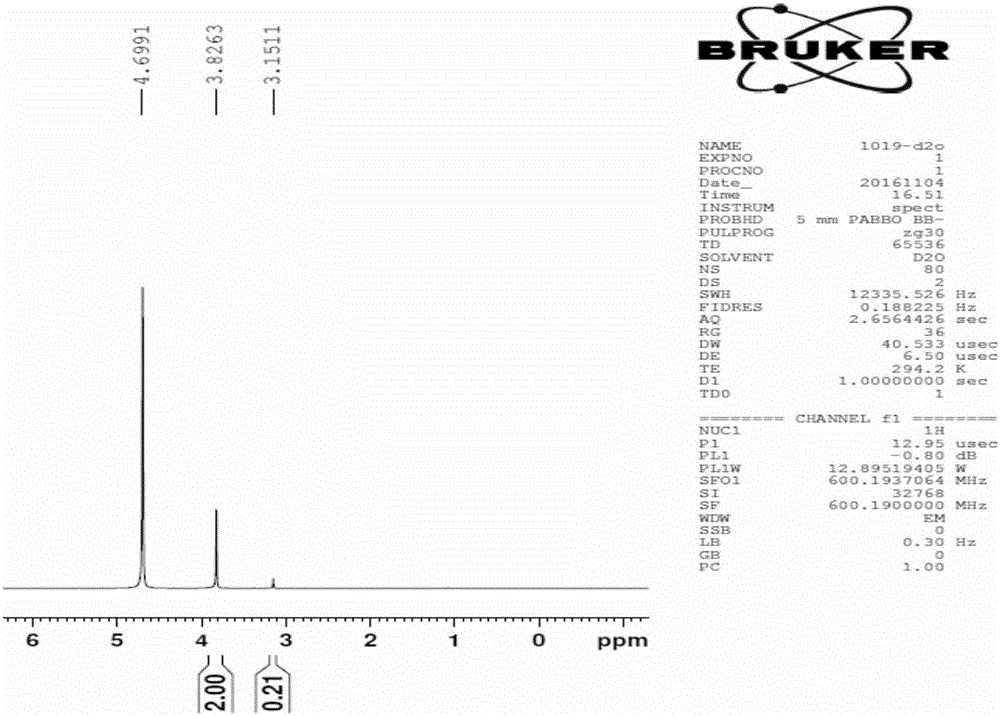
Hydrazine derivative, preparation method of hydrazine derivative and application of hydrazine derivative as sulphide ore surfactant
A technology of derivatives and carboxylic acid derivatives, used in organic chemistry, solid separation, flotation, etc., can solve the problems of increasing the hydrophilicity of non-molybdenum sulfide ore, and achieve a simple preparation method, high-efficiency separation, and mild reaction conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055]
[0056] In a 250mL three-necked flask, add 0.105mol of sodium hydroxide and dissolve it in 10ml of water. When the temperature is lowered to 0°C, add 0.1mol of hydrazine; use a dropping funnel to add 0.1mol of carbon disulfide dropwise, and keep the reaction temperature within the range of 0-5°C Stir until the carbon disulfide reaction in the solution is complete, then add dropwise 0.1mol sodium chloroacetate aqueous solution, and keep the temperature at 0-5°C; after the dropwise addition of sodium chloroacetate solution is completed, the temperature rises to room temperature, and the reaction continues for two hours to obtain surface activity The solvent solution product was distilled off under reduced pressure to remove the solvent to obtain a solid product with a yield of 81% based on hydrazine.
Embodiment 2
[0058]
[0059] In a 250 mL three-neck flask, dissolve 0.105 mol of potassium hydroxide in 100 ml of methanol and place in an ice-water bath, while adding 0.1 mol of hydrazine at one time; use a dropping funnel to drop 0.1 mol of carbon disulfide until the reaction of carbon disulfide is complete. Use a separating funnel to separate the yellow liquid in the lower layer, mix the yellow liquid solvent with 40% methanol solution; keep the temperature at 0-5°C, add 0.1mol methanol solution of chloroacetic acid dropwise, after the reaction is completed, distill under reduced pressure to obtain the product, The product was obtained after recrystallization, and the yield was 85% based on hydrazine.
Embodiment 3
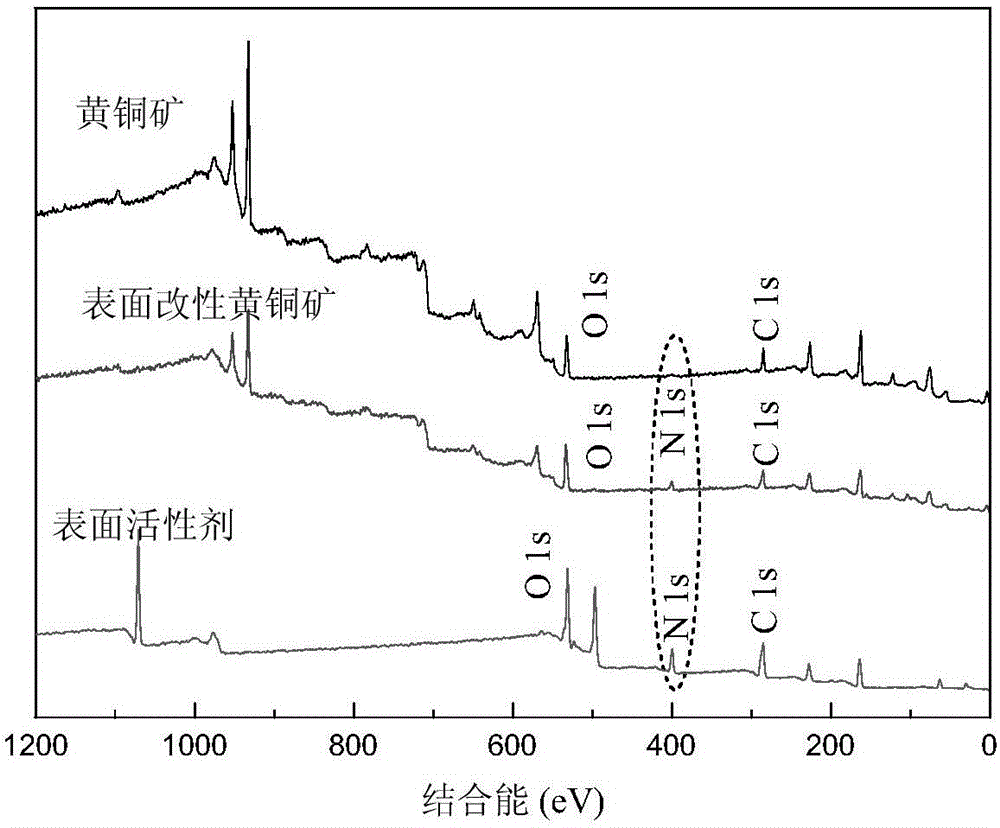
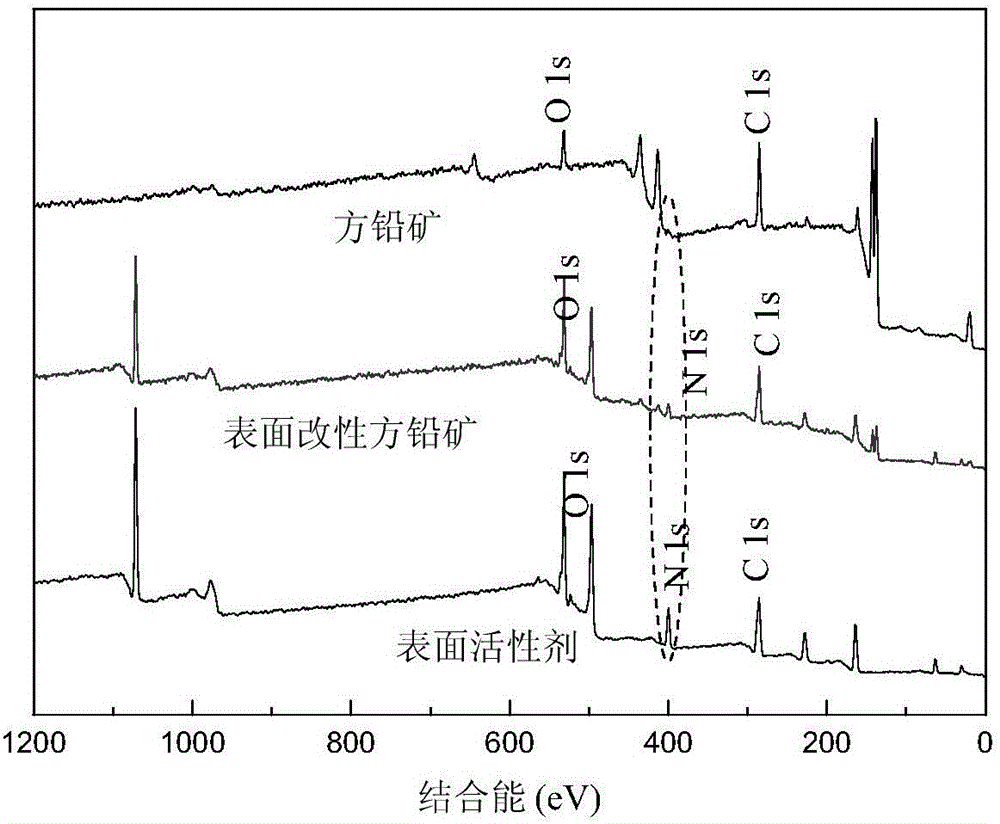
[0061] Modification of chalcopyrite by the surfactant product prepared in Example 1.
[0062] Add an appropriate amount of chalcopyrite powder into a 100 ml beaker, pour the prepared surfactant solution into the beaker, stir at room temperature for 2 hours, and filter to obtain modified chalcopyrite. The surface changes before and after modification are as follows figure 2 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com