Febuxostat 2-methylimidazole salt and preparation method thereof and pharmaceutical composition containing febuxostat 2-methylimidazole salt
A technology of methylimidazolium salt and febuxostat, which is applied in the field of medicine and chemical industry, can solve the problems of difficult crystal form control and low solubility of febuxostat, and achieve the effects of good crystal form stability, high solubility and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0044] The preparation method of febuxostat 2-methylimidazole salt of the present invention is relatively convenient.
[0045] (1) Dissolving febuxostat and 2-methylimidazole in an organic solvent respectively, and obtaining a corresponding solution;
[0046] In step (1), preferably, the molar ratio of the raw material febuxostat and 2-methylimidazole is (3:1) to (1:1); more preferably, the mol ratio of febuxostat and 2-methylimidazole is The molar ratio is (2.2:1)~(1.8:1);
[0047] Meanwhile, in step (1), preferably, the volume-to-mass ratio of the organic solvent to febuxostat or 2-methylimidazole is (5-50): 1; more preferably, the organic solvent to febuxostat or 2 - The volume-to-mass ratio of methylimidazole is (8-20): 1;
[0048] Meanwhile, the organic solvent is one of C1-C4 low-carbon alcohols, ethers, esters, ketones or nitriles, or a mixed organic solvent of several;
[0049] Preferably, the alcohol solvents are methanol, ethanol, propanol, isopropanol, butanol; e...
Embodiment 1
[0058] Dissolve 10.0 g of febuxostat in 100 mL of methanol; take 1.1 g of 2-methylimidazole and dissolve it in 10 mL of methanol, mix and stir the obtained two solutions, concentrate, cool to 10 °C for crystallization, and filter to obtain colorless crystals 3.3 gram;
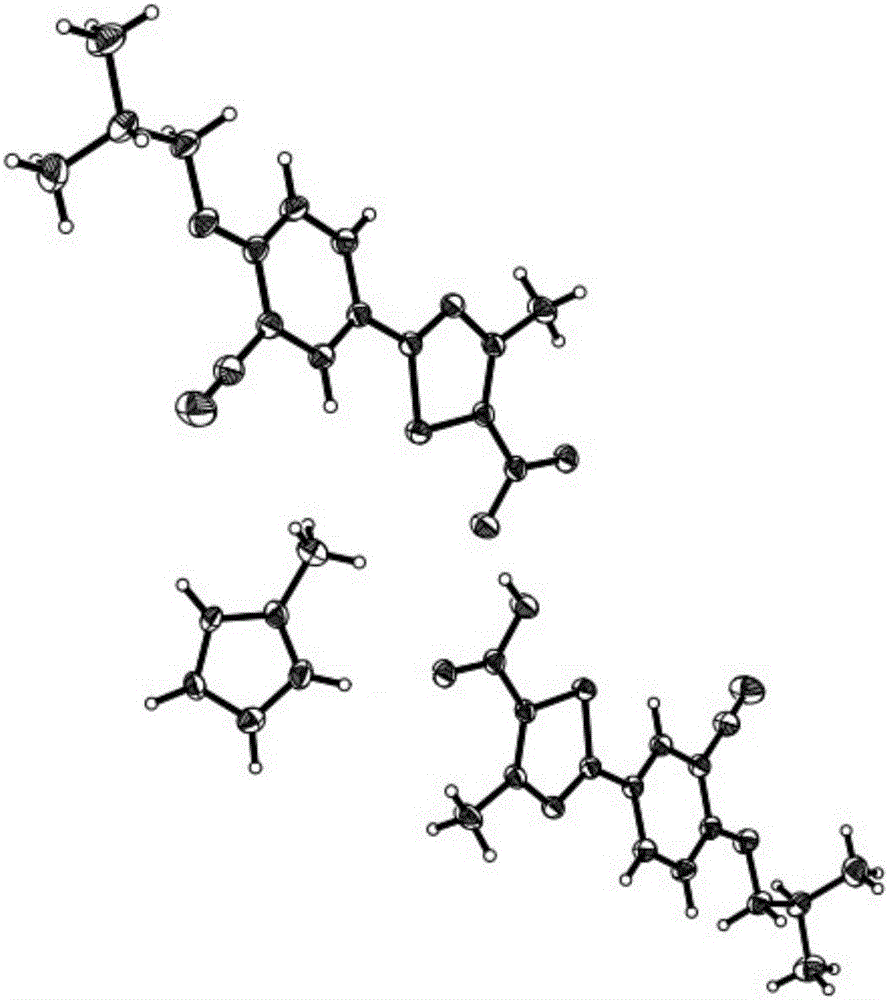
[0059] of the crystal 1 H NMR showed: δ: 8.22(s, 2H), 8.17(d, 2H), 7.33(d, 2H), 7.18(s, 2H), 3.98(d, 4H), 2.64(s, 6H), 2.44( s, 3H), 2.08 (hept, 2H), 1.01 (d, 12H);
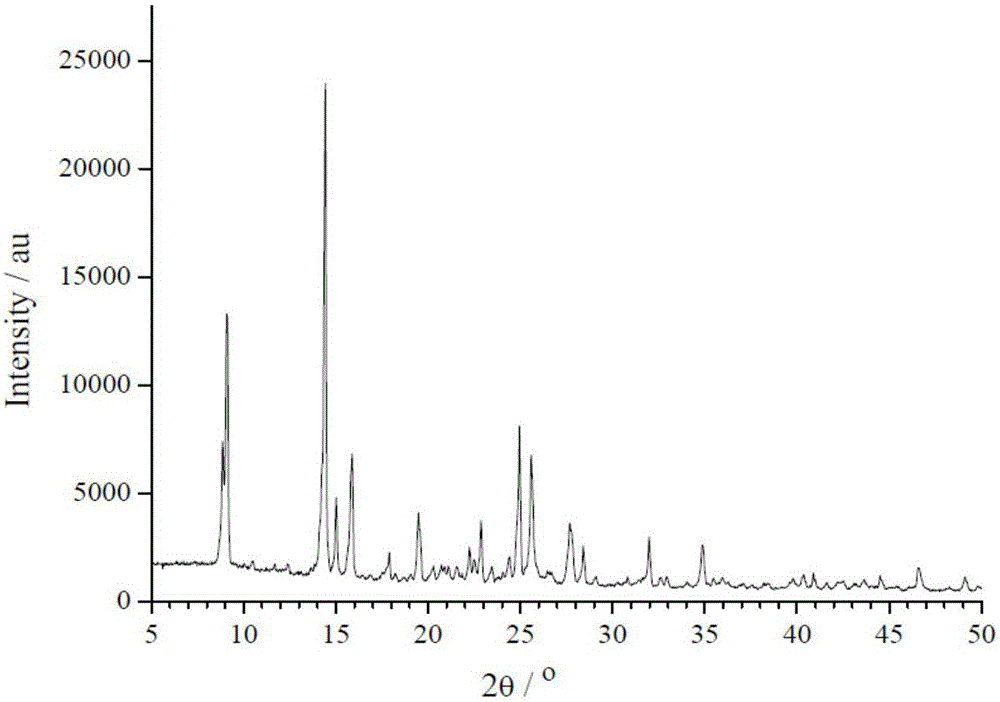
[0060] The powder XRD pattern of the crystal is as follows figure 2 shown, by figure 2 It can be seen that the obtained crystal has obvious diffraction peaks at 2θ angles of 8.82°, 9.13°, 14.38°, 15.88°, 19.53°, 24.90°, 25.61°, 27.70°, 32.00°, and 34.80°;
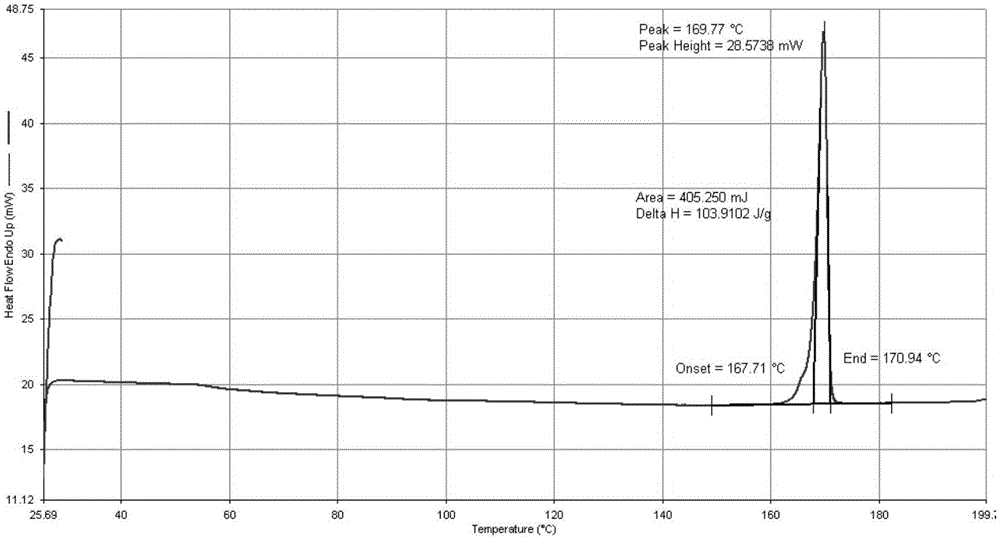
[0061] DSC of crystals as image 3 shown; by image 3 The melting point of this salt was found to be 169±2°C.
[0062] Infrared spectra of crystals such as Figure 4 shown, by Figure 4 The infrared spectrum diagram shows that the febuxostat 2-methylimidazole crystal of the present i...
Embodiment 2
[0065] Dissolve 6.3 g of febuxostat in 40 mL of acetonitrile, dissolve 1.2 g of 2-methylimidazole in 5 mL of acetonitrile, mix and stir the two solutions, concentrate, cool to 5 °C for crystallization, and filter to obtain colorless crystals 4.0 g.
[0066] The NMR, powder XRD diffraction and infrared spectrum tests of the crystal obtained in Example 2 are consistent with the results in Example 1, and its melting point is 169.3°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com