Bismuth-doped yellow tungstoborate phosphor as well as preparation method and application thereof
A yellow phosphor, tungsten borate technology, applied in chemical instruments and methods, luminescent materials, electrical components, etc., can solve problems such as the reduction of luminous efficiency, and achieve the effect of simple synthesis method, convenient large-scale production, and strong absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
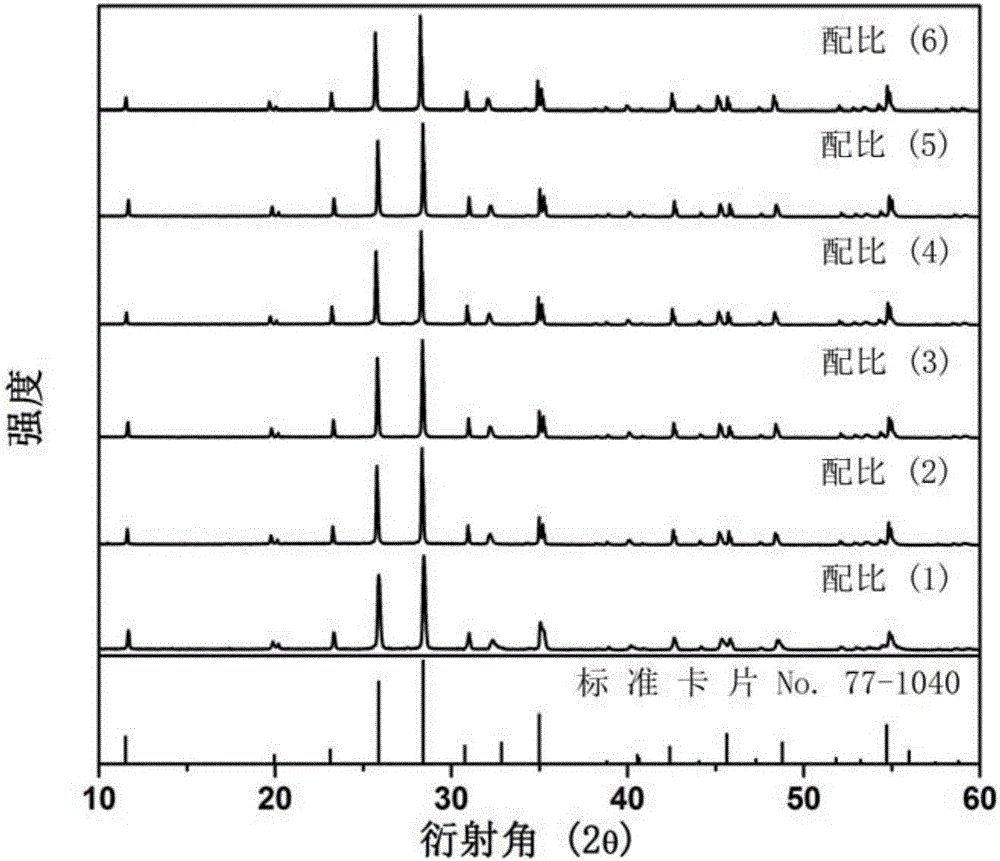
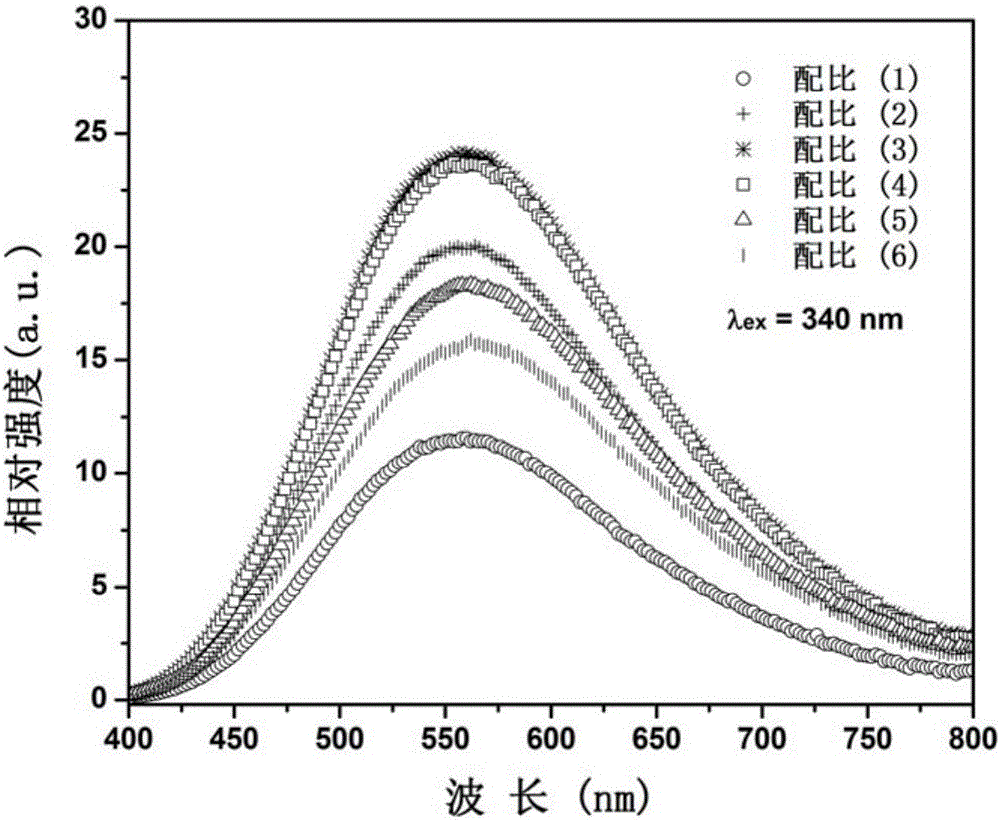
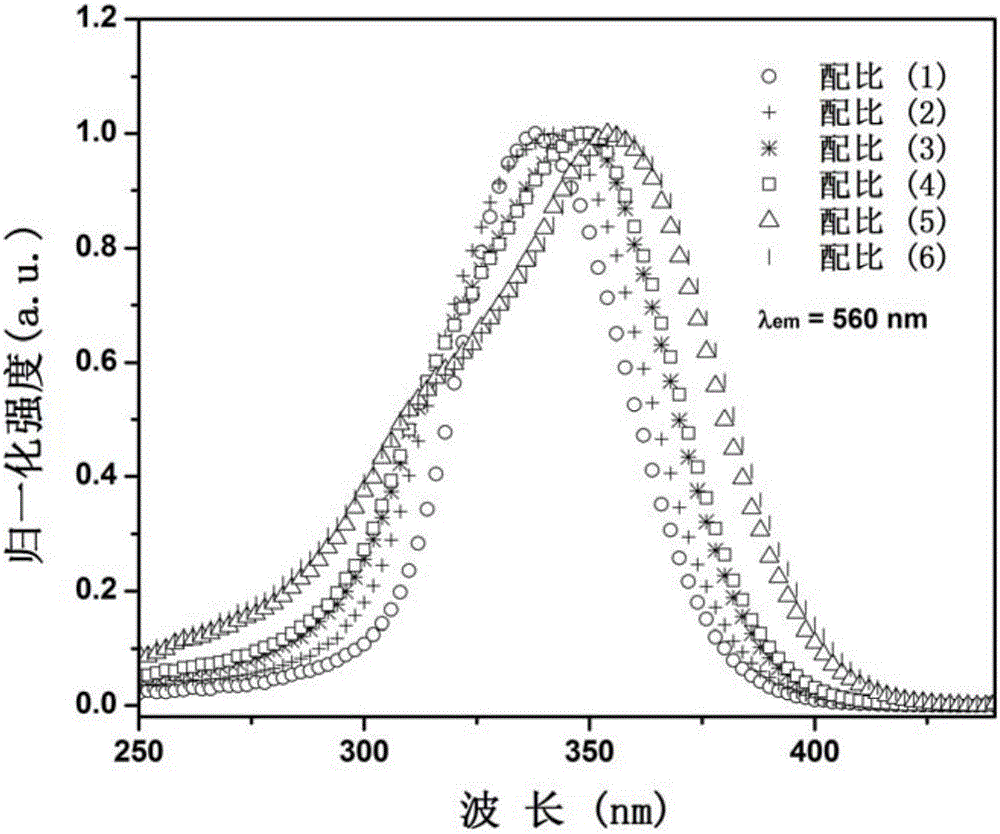
[0032] Select dilanthanum trioxide, boric acid, tungsten trioxide, and bismuth trioxide as starting compound raw materials, and weigh four kinds of compound raw materials respectively according to the stoichiometric ratio of each element, totally 6 groups, and the proportioning ratio is as follows:
[0033] (1) La:B:W:Bi=2.985:1:1:0.015, corresponding to x=0.5%;
[0034] (2) La:B:W:Bi=2.970:1:1:0.030, corresponding to x=1.0%;
[0035] (3) La:B:W:Bi=2.955:1:1:0.045, corresponding to x=1.5%;
[0036] (4) La:B:W:Bi=2.940:1:1:0.060, corresponding to x=2.0%;
[0037] (5) La:B:W:Bi=2.925:1:1:0.075, corresponding to x=2.5%;
[0038] (6) La:B:W:Bi=2.910:1:1:0.090, corresponding to x=3.0%;
[0039] After the mixture is ground and mixed evenly, it is loaded into a corundum crucible; the corundum crucible is placed in a corundum boat and placed in a high-temperature box-type electric furnace. Strictly control the heating rate, and pre-burn at 600°C for 4h. Cool to room temperature, ...
Embodiment 2
[0046] Select dilanthanum trioxide, boric acid, tungsten trioxide, and bismuth nitrate as starting compound raw materials, according to the molar ratio of each element La:B:W:Bi=2.955:1:1:0.045, corresponding to x=1.5%; Take four compound raw materials, grind and mix the mixture evenly, put it into a corundum crucible, place the crucible in a corundum boat, and put it into a high-temperature box-type electric furnace. Strictly control the heating rate and pre-burn at 650°C for 6h. Cool to room temperature, grind and mix; then calcinate at 1100°C for 12 hours, cool to room temperature with the furnace, and grind to obtain bismuth-doped tungstoborate yellow fluorescent material. XRD pattern analysis shows that it is La 3 BWO 9 crystal phase. The spectral properties of the fluorescent powder are similar to those of Example 1.
Embodiment 3
[0048] Select dilanthanum trioxide, boric acid, tungsten trioxide, and bismuth nitrate as starting compound raw materials, according to the molar ratio of each element La:B:W:Bi=2.910:1:1:0.090, corresponding to x=3.0%; Take four compound raw materials, grind and mix the mixture evenly, put it into a corundum crucible, place the crucible in a corundum boat, and put it into a high-temperature box-type electric furnace. Strictly control the heating rate and pre-burn at 600°C for 8h. Cool to room temperature, grind and mix; then calcinate at 1150°C for 8 hours, cool to room temperature with the furnace, and grind to obtain bismuth-doped tungstoborate yellow fluorescent material. XRD pattern analysis shows that it is La 3 BWO 9 crystal phase. The spectral properties of the fluorescent powder are similar to those of Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com