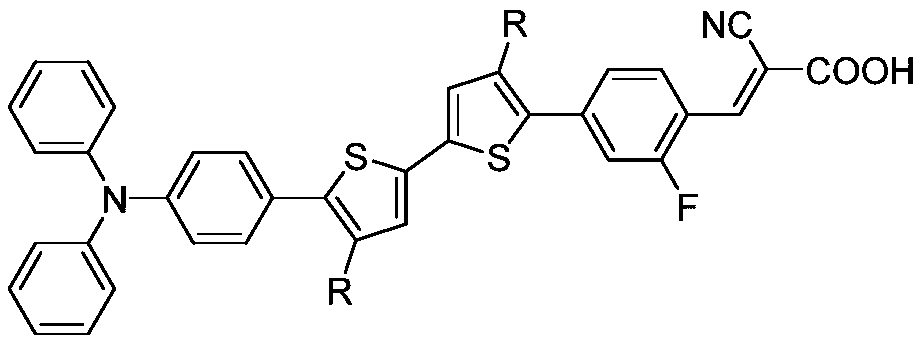
Alkyl bithiophene-2-fluorobenzene-bridged triphenylamine co-sensitizer and preparation method thereof
A technology of triphenylamine co-sensitizer and alkyl bithiophene, which is applied in the field of organic dyes, can solve the problems of narrow absorption band, large molar absorptivity, and weak absorption, and achieve the effects of enhanced absorption, strong absorption, and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
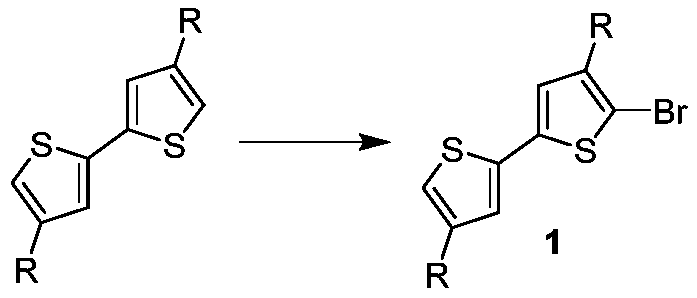
[0033] 1. Preparation of the compound of formula 1-1
[0034] Add 50mL of chloroform to the one-necked flask, then add 6.68g (20mmol) of 4,4'-dihexyl-2,2'-bithiophene, 1.74g (11mmol) of liquid bromine, react at 70°C for 4 hours, and cool to room temperature , then add 50mL saturated aqueous sodium bicarbonate solution, separate the layers, wash the organic phase with distilled water until neutral, dry with anhydrous magnesium sulfate, evaporate the solvent, and purify the residue by column chromatography (eluent is petroleum ether) to obtain The compound of formula 1-1 is 5.76g, and the yield is 70%. Its chemical reaction equation is as follows:
[0035]
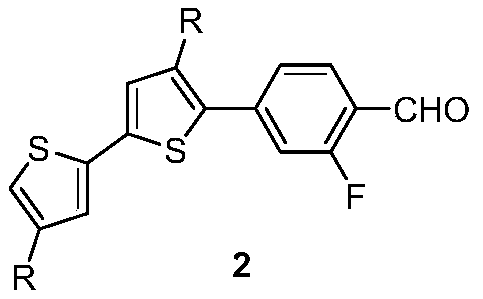
[0036] 2. Preparation of the compound of formula 2-1
[0037] Under nitrogen protection, add 32mL tetrahydrofuran and 8mL water to the three-neck round bottom flask, then add 4.12g (10mmol) formula 1-1 compound, 1.85g (11mmol) 3-fluoro-4-formylphenylboronic acid, 0.8g ( 11mmol) sodium bicarbonate, 0.4g (0.50mmol) tetra...
Embodiment 2
[0050] In step 1 of Example 1, the 4,4'-dihexyl-2,2'-bithiophene used was replaced with equimolar 4,4'-dimethyl-2,2'-bithiophene, and other steps Same as in Example 1, a methylbithiophene-2-fluorobenzene-bridged triphenylamine sensitizer with the following structural formula was prepared.
[0051]
Embodiment 3
[0053] In step 1 of Example 1, the 4,4'-dihexyl-2,2'-bithiophene used was replaced with equimolar 4,4'-didecyl-2,2'-bithiophene, and the other steps In the same manner as in Example 1, a decylbithiophene-2-fluorobenzene-bridged triphenylamine sensitizer with the following structural formula was prepared.
[0054]
[0055] In order to prove the beneficial effect of the present invention, the inventor dissolves the co-sensitizers prepared in Examples 1 to 3 in dichloromethane respectively to form 10 -5 The dichloromethane solution of mol / L co-sensitizer adopts UV-Vis spectrometer (Hitachi U-3900 / 3900H) to record the ultraviolet absorption spectrum of co-sensitizer, and its maximum absorption wavelength range is 380~420nm, and molar extinction coefficient range is 30000~50000M -1 cm -1 .
[0056] In addition, the inventors used the co-sensitizers prepared in Examples 1-3 to prepare dye-sensitized solar cells. The specific preparation method is as follows:
[0057] TiO 2 T...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molar extinction coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com