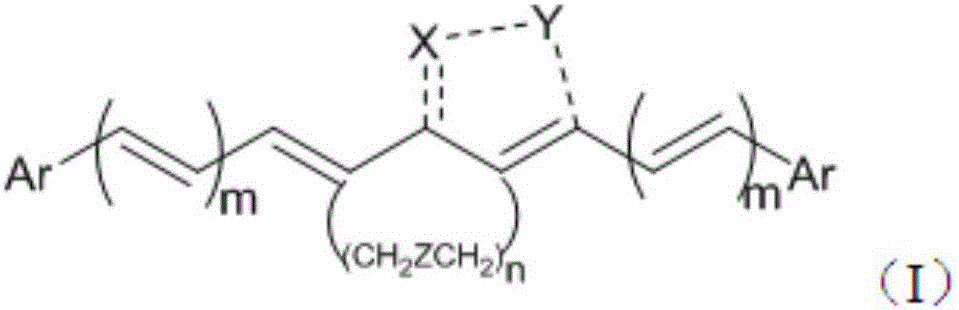
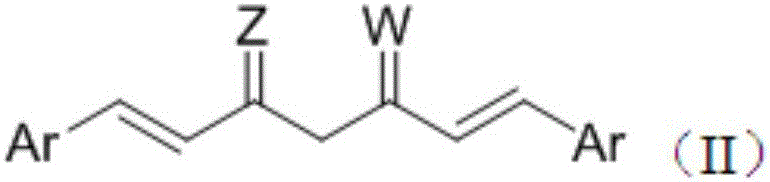
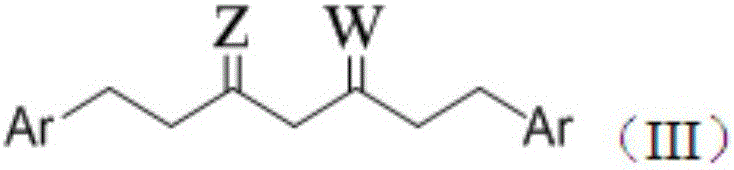
Bis-substituted aryl compounds and application thereof
A double-substitution and compound technology, applied in the field of pharmaceutical compounds, can solve problems such as difficulty in clinical drug selection and increased resistance of fungi to drugs, and achieve the effect of reversing drug resistance and improving sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Example 1: Preparation of (1E,4E)-1,5-bis(thiophen-2-yl)penta-1,4-dien-3-one
[0081]
[0082] Take acetone (10mmol), add ethanol 20ml, after mixing, slowly add 10% to 50% NaOH aqueous solution (40mmol) and 2-thiophene carboxaldehyde (20mmol) at room temperature, stir at room temperature until the reaction is complete, then pour the reaction solution into 200ml A yellow solid was precipitated in ice water, filtered and dried, and the crude product was recrystallized from ethanol to obtain a yellow solid with a melting point of 116-118°C and a yield of 63%. ESIMS m / z:247.0[M+H] + .
Embodiment 2
[0083] Example 2: Preparation of (1E,4E)-1,5-bis(3,4-dihydroxyphenyl)penta-1,4-dien-3-one
[0084]
[0085] Take acetone (10mmol), add ethanol 20ml, after mixing, slowly add 10% to 50% NaOH aqueous solution (40mmol) and veratraldehyde (20mmol) at room temperature, stir at room temperature until the reaction is complete, then pour the reaction solution into 200ml of ice In water, a yellow solid was precipitated, filtered and dried, and the crude product was recrystallized with ethanol to obtain a yellow solid. After drying, take the yellow solid (1mmol), add 20ml of dry dichloromethane, and slowly add BBr at -15°C 3 (12mmol), after the dropwise addition was completed, it was moved to room temperature and stirred until the reaction was completed, and treated with water, a solid was precipitated, filtered, dried, and the crude product was recrystallized with ethanol to obtain a red solid. ESIMS m / z:299.1[M+H] + .
Embodiment 3
[0086] Example 3: Preparation of 2,5-bis((E)-3,4-dihydroxybenzylidene)cyclopentan-1-one
[0087]
[0088] Take cyclopentanone (10mmol), add 20ml of ethanol, after mixing, slowly add 10% to 50% NaOH aqueous solution (40mmol) and veratraldehyde (20mmol) at room temperature, stir at room temperature until the reaction is complete, then pour the reaction solution into In 200ml of ice water, the yellow solid precipitated was filtered and dried, and the crude product was recrystallized with ethanol to obtain a yellow solid. After drying, the yellow solid (1mmol) was taken, and 20ml of dry dichloromethane was added, and BBr was slowly added dropwise at -15°C. 3 (12mmol), after the dropwise addition was completed, it was moved to room temperature and stirred until the reaction was completed, treated with water, and a solid was precipitated, filtered, dried, and the crude product was recrystallized with ethanol. ESIMS m / z:325.9[M+H] + .Example 4: Preparation of 2,5-bis((E)-3,4-dihy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com