Method for preparing chiral diphenyl pyrrolidone and intermediate compounds
A biphenylpyrrolidone and chirality technology is applied in the field of preparation of chiral biphenylpyrrolidone and achieves the effect of short route and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
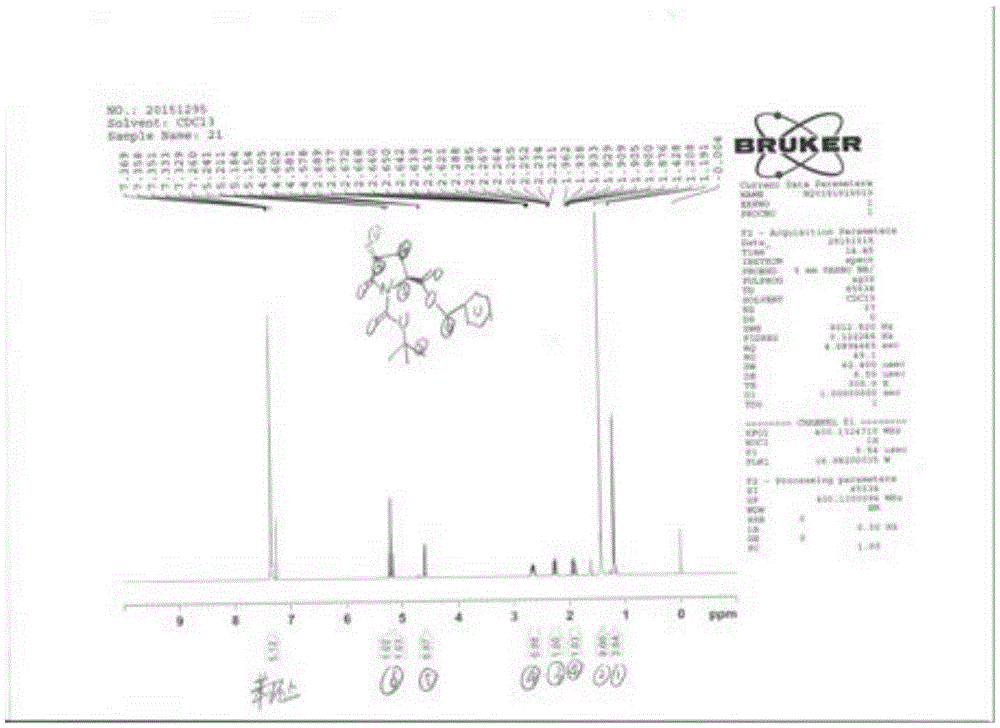
[0037] Embodiment 1: the synthesis of compound 2
[0038] Dissolve 31.9g (0.1mol) of Boc-L-benzyl pyroglutamate in dry tetrahydrofuran, cool to -80°C, add 110ml of 1N LiHMDS solution dropwise, stir for 30 minutes after dropping, then add 28.4 g (0.2 mol) of methyl iodide, after dropping, stirred for about 1 hour, then naturally warmed to room temperature, and stirred overnight. Under cooling in an ice bath, 200 ml of saturated ammonium chloride solution was added, extracted with ethyl acetate, and the organic layer was washed with water until neutral, dried and concentrated to dryness under reduced pressure. 600g of silica gel column chromatography, eluting with ethyl acetate: n-hexane = 1:4, gave 21.6g of intermediate. Yield 64.9%, m / z 356.15 (100%, M+Na+ peak), 234.11 (50%, off Boc peak). 1H-NMR: see appendix figure 1 .
Embodiment 2
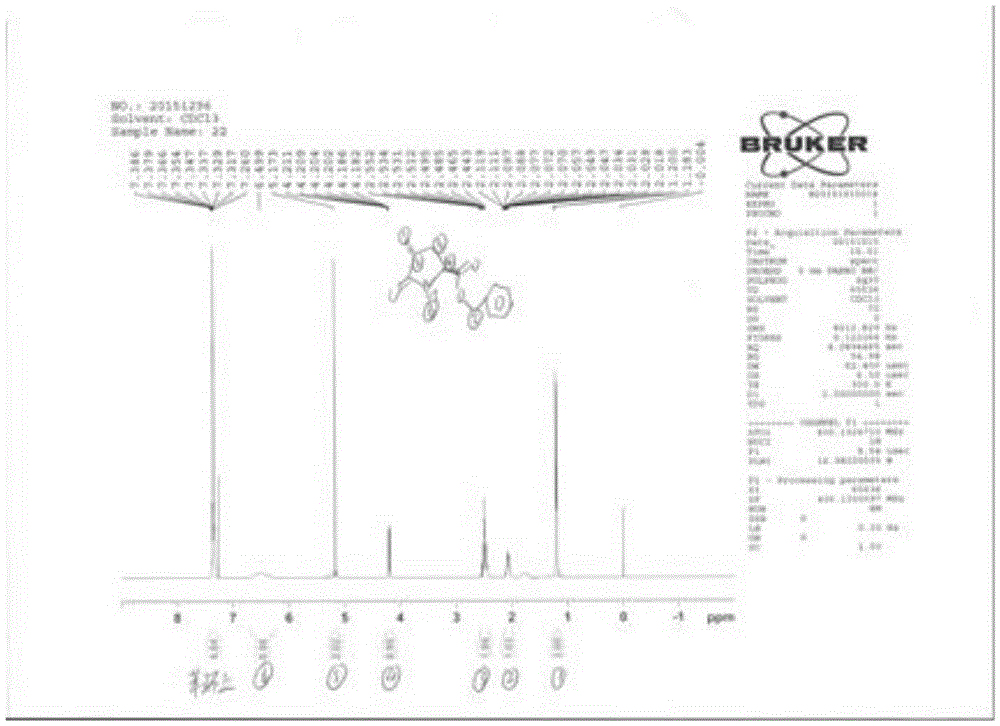
[0039] Embodiment 2: the synthesis of compound 3
[0040] Dissolve 17.7g (0.05mol) of CBZ-L-benzyl pyroglutamate in dry tetrahydrofuran, cool to -80°C, add 75ml of 1N LiHMDS solution dropwise, stir for 30 minutes after dropping, then add dropwise 14.2 g (0.1 mol) of iodomethane was added after dropping, stirred for about 1 hour, warmed up to room temperature naturally, and stirred overnight. Under cooling in an ice bath, 100 ml of saturated ammonium chloride solution was added, extracted with ethyl acetate, and the organic layer was washed with water until neutral, dried and concentrated to dryness under reduced pressure. Mass spectrum showed m / z 390.16 (M+Na+ peak).
Embodiment 3
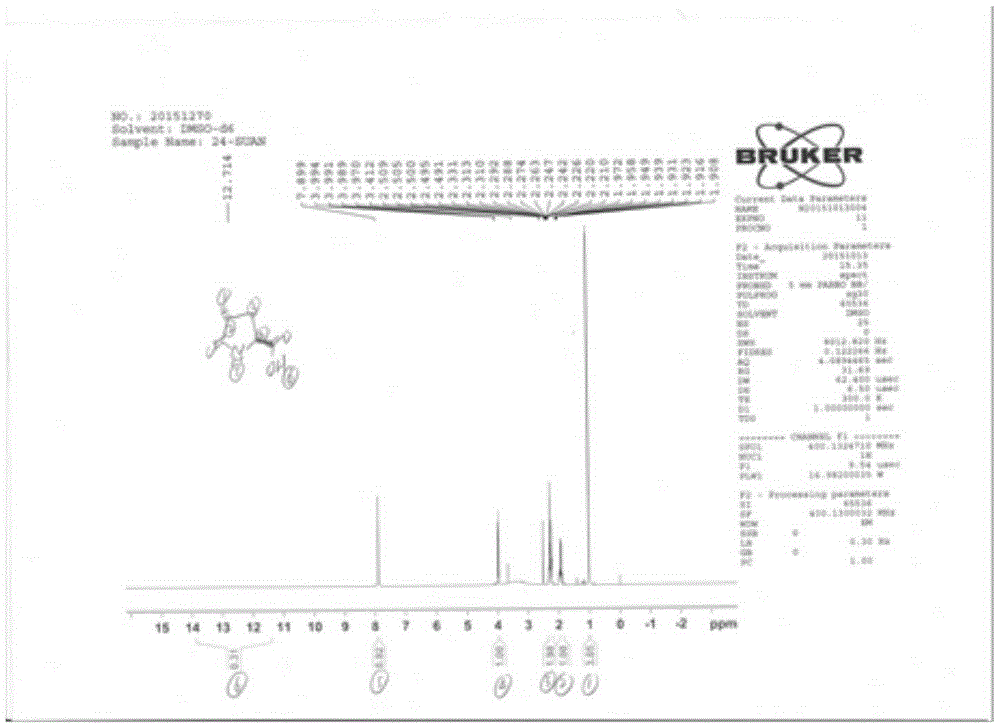
[0041] Embodiment 3: the synthesis of compound 4
[0042] Dissolve 40g (0.12mol) of intermediate 2 in dichloromethane, add 4.4ml of trifluoroacetic acid, stir at room temperature for 2 hours, and remove the solvent to dryness under reduced pressure. Add methanol, 1g of 10% palladium carbon, and hydrogenate in a 5Kg / cm2 hydrogenation reactor until the reaction is complete. Filter and concentrate to dryness under reduced pressure. Crystallization with ethyl acetate gave white solid 15.8g, yield 92.1%, m / z ES+144.06 (100% M+H+ peak), 166.03 (M+Na+ peak); ES-142.02 (M-H+ peak)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com