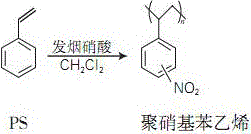
Preparation method of polynitrostyrene
A technology of nitrostyrene and fuming nitric acid, applied in the field of organic polymers, can solve the problems of low nitration rate, small reaction interface, insoluble final product, etc., achieve high nitro group introduction rate, short reaction time, and simple method Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Add 20 parts by weight of a mixture of fuming nitric acid and concentrated sulfuric acid (ratio: 1:1), 40 parts by weight of dichloromethane into the dried mixing tank, then start stirring for 1 hour, cool to 0°C, and slowly drop in quantitative 100 parts by weight of styrene monomer were stirred for 3 hours, and absolute ethanol was added dropwise to terminate the reaction, and the product was filtered to obtain a product of colorless crystals. The product was washed with deionized water and dried to constant weight.
[0016] The yield calculated by weighing method was 87%, and the nitro group introduction rate was 96%.
Embodiment 2
[0018] Add 30 parts by weight of a mixture of fuming nitric acid and concentrated sulfuric acid (ratio: 1:2) and 30 parts by weight of dichloromethane into the dried mixing tank, then start stirring for 1 hour, cool to 0°C, and slowly drop in quantitative 100 parts by weight of styrene monomer were stirred for 3 hours, and absolute ethanol was added dropwise to terminate the reaction, and the product was filtered to obtain a product of colorless crystals. The product was washed with deionized water and dried to constant weight.
[0019] The yield calculated by weighing method was 92%, and the nitro introduction rate was 98%.
Embodiment 3
[0021] Add 30 parts by weight of a mixture of fuming nitric acid and concentrated sulfuric acid (ratio: 1:3) and 30 parts by weight of dichloromethane into the dried mixing tank, then start stirring for 1 hour, cool to 0°C, and slowly drop in quantitative 100 parts by weight of styrene monomer were stirred for 3 hours, and absolute ethanol was added dropwise to terminate the reaction, and the product was filtered to obtain a product of colorless crystals. The product was washed with deionized water and dried to constant weight.
[0022] The yield calculated by the weighing method was 96%, and the nitro group introduction rate was 98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com