Lipid polymer and preparation method thereof, and method for introducing gene editing plasmid into eukaryotic cells by adopting lipid polymer
A eukaryotic cell and gene editing technology, applied in the field of biomedicine, can solve the problems of cationic liposome intolerance to serum, high eukaryotic cell toxicity, and low eukaryotic cell survival rate, achieving low toxicity, high import rate, The effect of reducing damage rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0051] The invention provides a kind of preparation method of lipopolymer, specifically comprises the following steps:
[0052] S1: Take amphoteric phospholipid solution, anionic phospholipid solution and cholesterol solution;
[0053] S2: mixing the amphoteric phospholipid solution, the anionic phospholipid solution and the cholesterol solution and stirring evenly to obtain a mixture A;
[0054] S3: Dry the mixture A under the protection of an inert gas at a drying temperature of 50-60° C. until a lipid film is formed to stop drying;
[0055] S4: adding buffer to the lipid film to hydrate the suspension to form suspended liposomes;
[0056] S5: The suspended liposomes pass through a filter with a fixed pore size while being heated, and then extruded into liposomes with uniform diameter by one of the ultrasonic method, microporous extrusion method, dialysis method or ethanol injection method. The diameter of the plastid is 50nm-2000nm, and the extrusion temperature is 45-55°...
Embodiment 1
[0065] Used instrument and reagent among the embodiment 1 are as follows:
[0066] The electrophoresis apparatus was Mini-Sub Cell GT from Bio-Rad. Avanti mini extruder is produced from Avantipolar lipids. The microplate reader was iMark from Bio-Rad. The microscope was a DM IL LED Fluo from Leica.
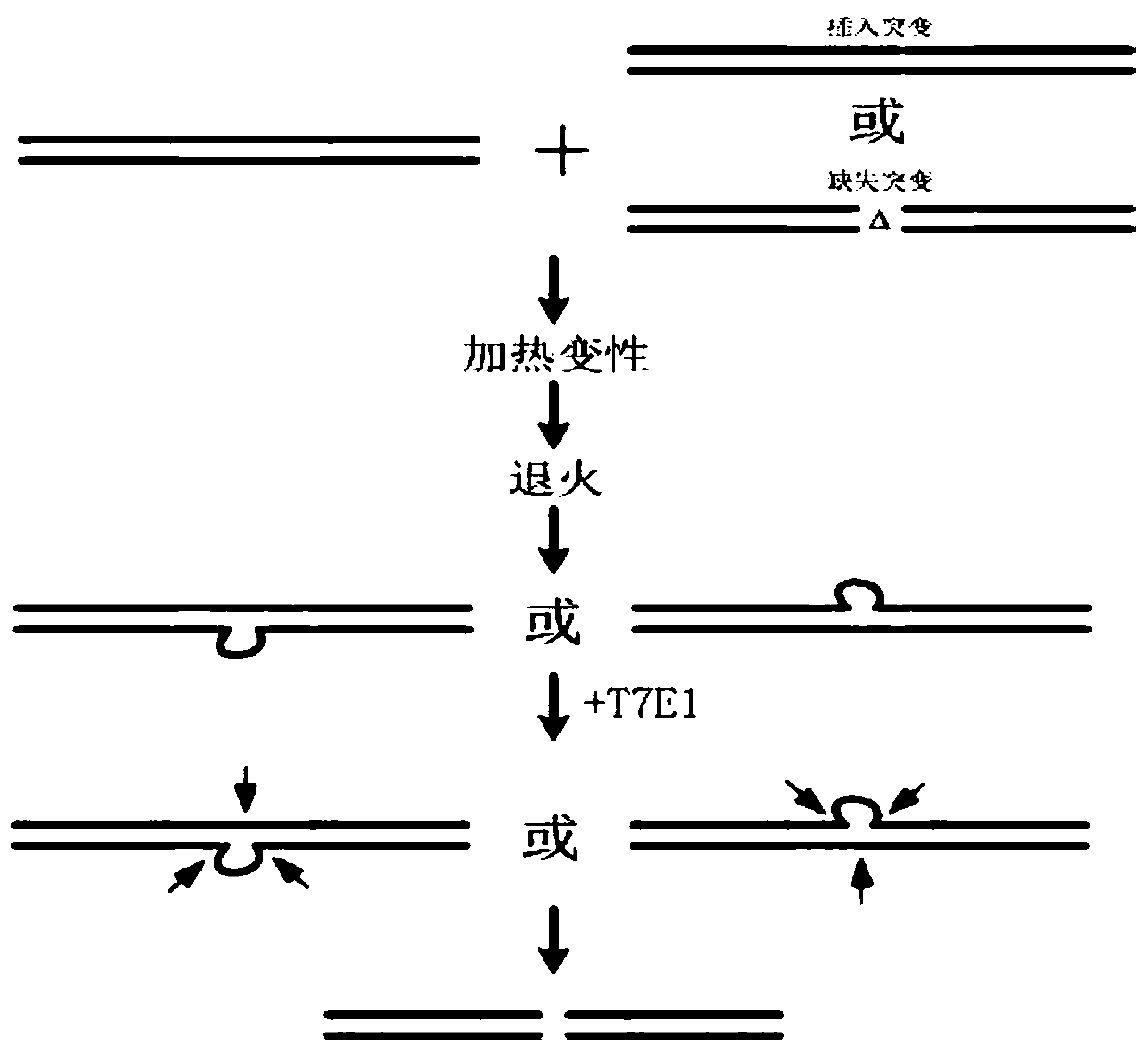
[0067] Primers were synthesized by Shanghai Sangong. The gene editing plasmid pX330 was from Addgene. Phospholipids are from Avanti polarlipids. Polyethyleneimine was from Basf. Cationic liposome Lipo8000 is from Biyuntian. WST and 1-methoxy-PMS in CCK8 staining solution were from SIGMA. HEK293 eukaryotic cells were from ATCC. T7E1 enzyme was from NEB. Other conventional reagents are of high purity. High-purity water meets the MilliQ standard.
[0068] 1. Preparation of gene editing plasmids
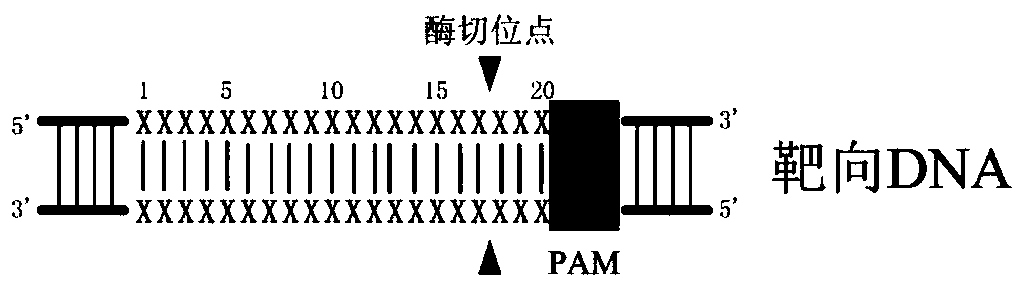
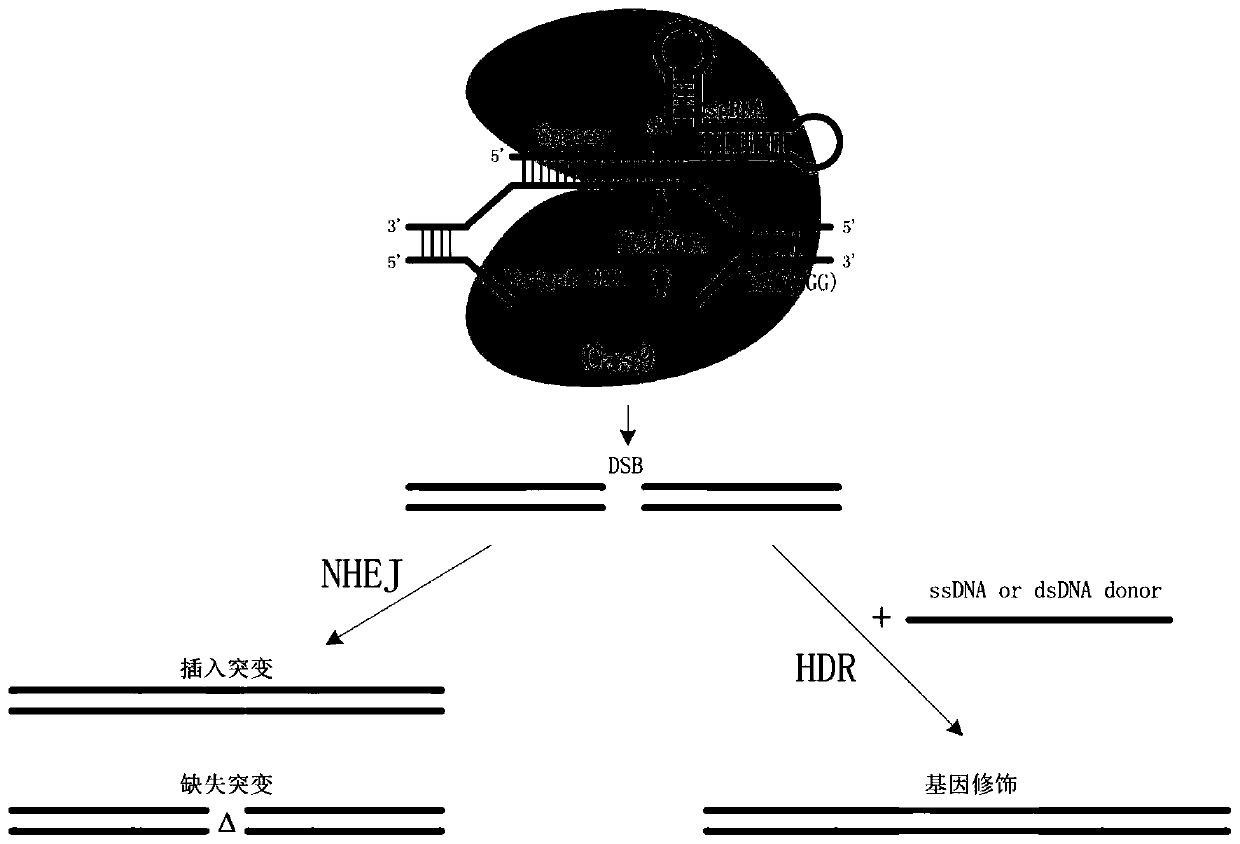
[0069] The gene editing plasmids pX330 / sgCLTA, pX330 / sgVEGF and pX330 / sgAAVS1 targeting the human CLTA gene, VEGF gene and AAVS1 gene were constructed using the gene editing plas...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com