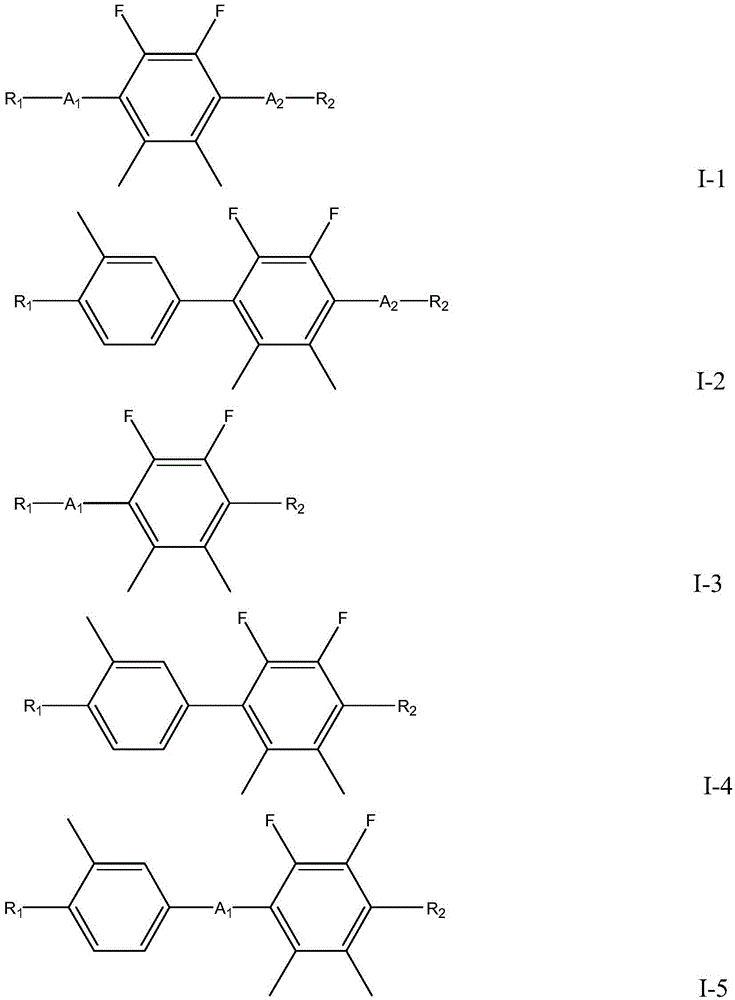
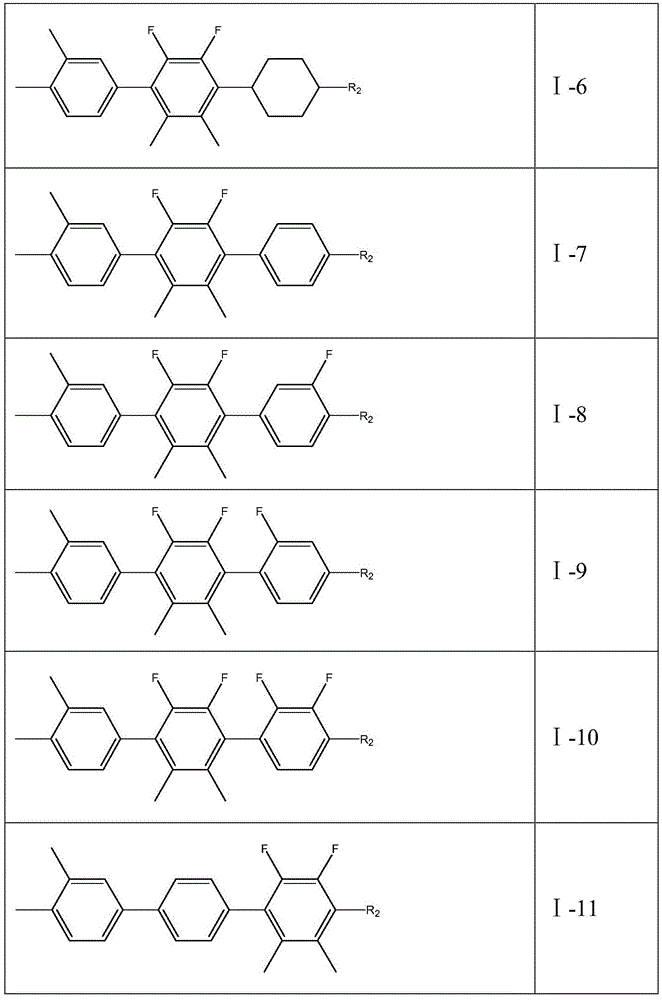
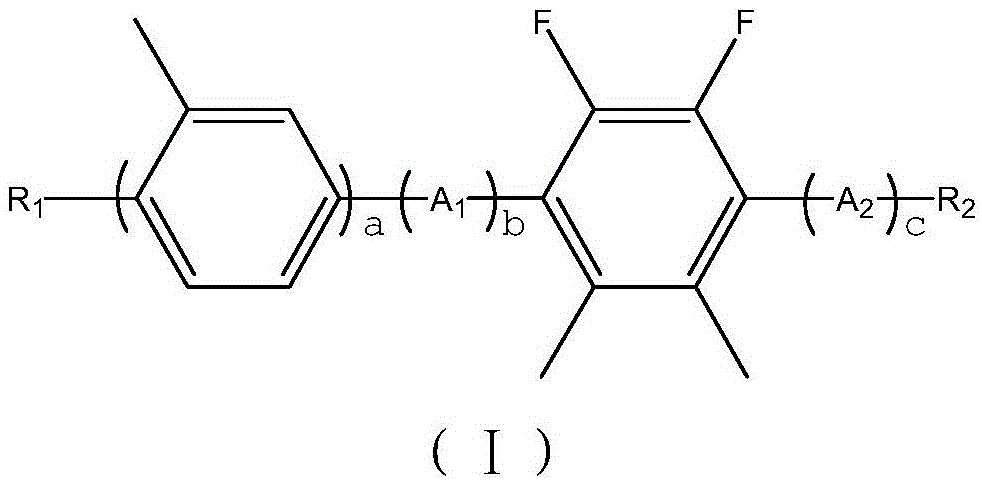2,3-difluoro-5, 6-dimethylphenyl-containing liquid crystal compound as well as preparation method and application thereof
A liquid crystal compound and compound technology, applied in the preparation of organic compounds, chemical instruments and methods, organic chemistry, etc., can solve the problems of TFT-LCD response is not fast enough, voltage is not low enough, charge retention rate is not enough, etc., to improve the response speed , the effect of low rotational viscosity and high elastic constant
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0098] Synthesis of (LC-01)
[0099]
[0100] 1) Synthesis of 2,3-difluoro-4,5,6-trimethylphenylboronic acid (compound 1-2)
[0101] Add 31.2g of 3,4-difluoro-5,6-dimethyltoluene (compound 1-1, 0.2mol) and 1000ml of tetrahydrofuran into a 2L dry and clean three-necked flask. ℃, add 80ml of butyllithium dropwise, and react for 1 hour under temperature control after dropping, add 32g of trimethyl borate (0.30mol) dropwise, after dropping, control temperature at -75°C~-85°C for 30 minutes, then naturally heat up to -20°C , Add dropwise 600ml aqueous hydrochloric acid. Separation, the aqueous phase was extracted twice with 400ml×2 ethyl acetate, the organic phases were combined, and spin-dried to obtain a white solid, the liquid phase purity (LC) was 99.0%, the theoretical yield was 40.0g, the actual yield was 36.0g, and the yield was 90%;
[0102] 2) Synthesis of 1,2-difluoro-3,4,5-trimethyl-6-(4-propyl-cyclohexyl)benzene (LC-01)
[0103] Add 40mL of absolute ethanol, 800...
Embodiment 2
[0108] Synthesis of (LC-02)
[0109] The route is as follows:
[0110]
[0111] 1) Synthesis of 2,3-difluoro-5,6-dimethylphenylboronic acid (compound 2-2)
[0112]Add 14.2g of 3,4-difluoro-5-methyltoluene (0.1mol) and 500ml of tetrahydrofuran to a 1L dry and clean three-necked flask, protect with nitrogen, cool down to -75°C to -85°C with liquid nitrogen, and add 40ml of butyl Lithium, temperature control reaction for 1h, dropwise add 15.6g trimethyl borate (0.15mol), dropwise, temperature control -75℃~-85℃ for 30 minutes, then naturally warm to -20℃, dropwise add 300ml hydrochloric acid aqueous solution . Separation, the aqueous phase was extracted twice with 200ml×2 ethyl acetate, the organic phase was combined, and spin-dried to obtain 16.4g of white solid, the liquid phase purity (LC) was 98%, the theoretical yield was 18.6g, and the yield was 88%;
[0113] 2) Synthesis of 2,3-difluoro-5,6-dimethyl-2',3'-difluoro-4'-butoxybiphenyl (compound 2-3)
[0114] Add 40mL ...
Embodiment 3
[0125]
[0126] 1) Synthesis of 3,4-dimethylphenylboronic acid (compound 3-2)
[0127] Add 18.5g of 3,4-difluorobromobenzene (compound 3-1, 0.1mol) and 500ml of tetrahydrofuran into a 1L dry and clean three-necked flask, and react with 2.64g of magnesium chips (0.11mol) under nitrogen protection, reflux for 1h, and then cool down To -30--40 degrees, dropwise add 16g of trimethyl borate (0.15mol), after dropping, raise the temperature to -10 degrees, then add dropwise 200ml of hydrochloric acid aqueous solution. Separation, the aqueous phase was extracted twice with 100ml×2 ethyl acetate, the organic phases were combined, and spin-dried to obtain a white solid, the liquid phase purity (LC) was 99.0%, the theoretical yield was 15g, the actual yield was 13g, and the yield was 86%;
[0128] 2) Synthesis of 3-fluoro-4-bromo-3',4'-dimethylbiphenyl (compound 3-3)
[0129] Add 26mL of absolute ethanol, 50mL of toluene and 50mL of water into a 200mL three-necked flask, start stirri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com