A kind of preparation method of drug sustained-release film
A slow-release film and drug technology, used in medical science, bandages, absorbent pads, etc., can solve the problems of unfavorable and comfortable dressings, insufficient hygroscopicity, and low elongation at break, and achieve excellent biomechanics and moisture absorption properties. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] A kind of preparation method of drug sustained-release membrane, according to the following steps:
[0027] (1) Preparation of regenerated silk fibroin solution:
[0028] Cut high-quality silkworm cocoons into spiral shapes, degummed and dried, then dialyzed, centrifuged, and filtered to obtain a certain concentration of regenerated silk fibroin, and set aside at 4 °C;
[0029] (2) Preparation of sodium alginate solution:
[0030] Prepare 0.8% sodium alginate solution with distilled water, stir for 10 h, and set aside at 4 °C;
[0031] (3) Preparation of drug sustained release film:
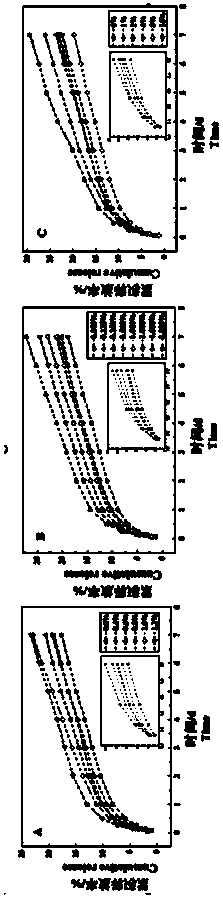
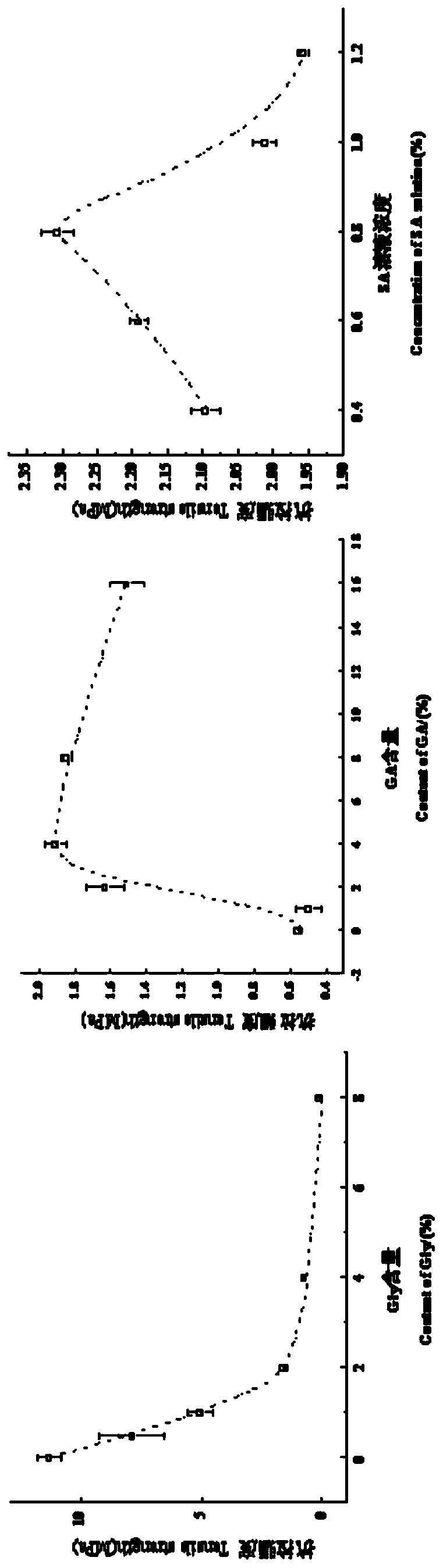
[0032] Table 1 Addition amount of sodium alginate, glycerol and glutaraldehyde
[0033]
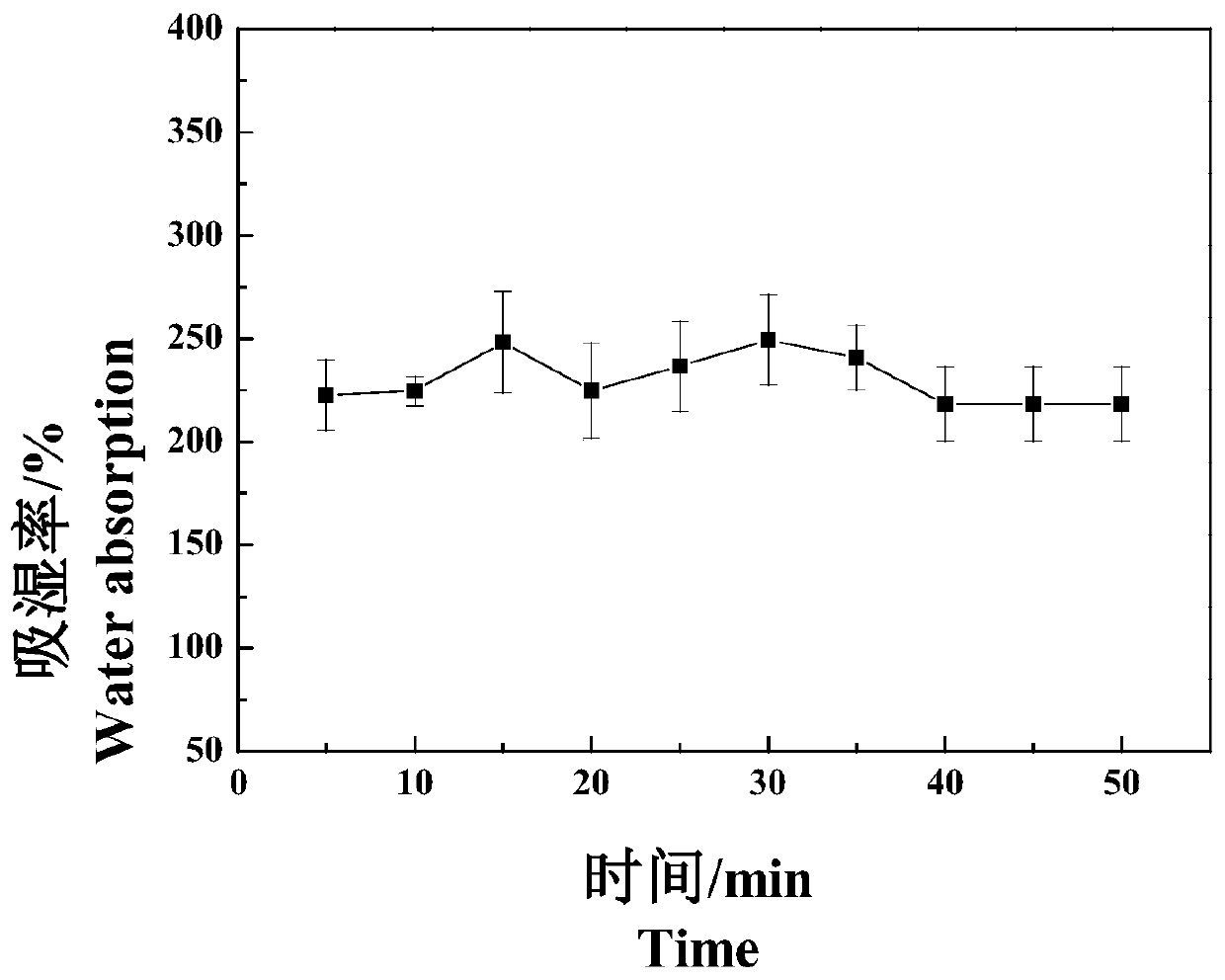
[0034] Mix silk fibroin with 0.8% sodium alginate at 25 °C, stir at 180 rpm for 25 min; add 75 % glycerol to a volume ratio of 2.0%, at 25 °C, stir at 180 rpm for 15 min, adjust with 0.1 M HCl After the pH reached 6.8, glutaraldehyde was added, and the final volume ratio was shown in Table 1. S...
Embodiment 2
[0047] A kind of preparation method of drug sustained-release membrane, according to the following steps:
[0048] (1) Preparation of regenerated silk fibroin solution:
[0049] Cut high-quality silkworm cocoons into spiral shapes, degummed and dried, then dialyzed, centrifuged, and filtered to obtain a certain concentration of regenerated silk fibroin, and set aside at 4 °C;
[0050] (2) Preparation of sodium alginate solution:
[0051] Sodium alginate solutions of different concentrations were prepared with distilled water, stirred for 10 h, and set aside at 4 °C;
[0052] (3) Preparation of drug sustained release film:
[0053] Table 2 Addition amount of sodium alginate, glycerol and glutaraldehyde
[0054]
[0055] Mix silk fibroin with 0.8% sodium alginate at 180 rpm for 25 minutes; add 75% glycerin to the final volume ratio in Table 2, stir for 15 minutes, adjust the pH to 7.4, and then add glutaraldehyde. 4.0%, stirred for 40 minutes, continued to add the model d...
Embodiment 3
[0063] A kind of preparation method of drug sustained-release membrane, according to the following steps:
[0064] (1) Preparation of regenerated silk fibroin solution:
[0065] Cut high-quality silkworm cocoons into spiral shapes, degummed and dried, then dialyzed, centrifuged, and filtered to obtain a certain concentration of regenerated silk fibroin, and set aside at 4 °C;
[0066] (2) Preparation of sodium alginate solution:
[0067] Sodium alginate solutions of different concentrations were prepared with distilled water, stirred for 10 h, and set aside at 4 °C;
[0068] (3) Preparation of drug sustained release film:
[0069] Table 3 Addition amount of sodium alginate, glycerol and glutaraldehyde
[0070]
[0071] Mix silk fibroin with 0.8% sodium alginate at 180 rpm and stir for 25 minutes; add 75% glycerin to a volume ratio of 2.0%, stir for 15 minutes, adjust the pH to 7.4 and add glutaraldehyde, the final volume ratio is Table 1, stir for more than 2 hours, con...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com