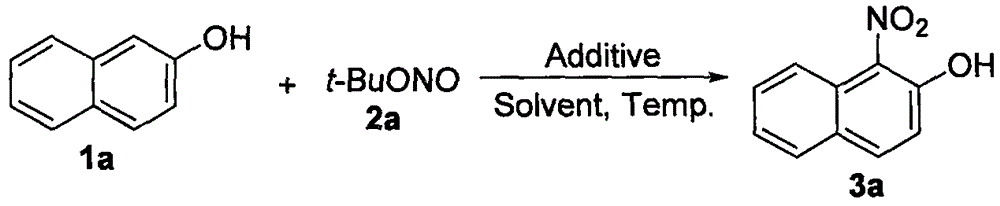
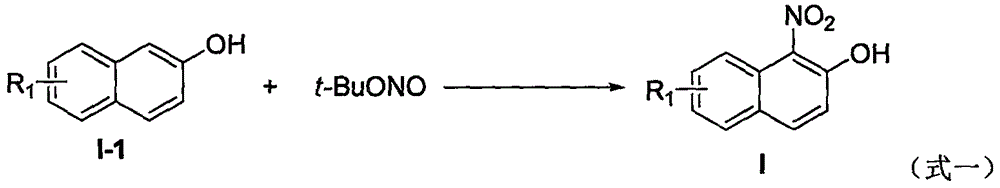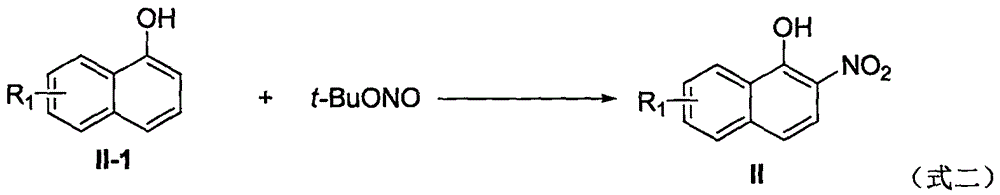Preparation method of 1-nitro-2-naphthol derivative
A technology of naphthol derivatives and nitro, which is applied in the field of preparation of nitronaphthol derivatives, can solve problems such as inappropriate preparation of nitronaphthol derivatives, and achieve the effects of increasing yield and improving selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 17
[0040]Example 17 Synthesis of 6-bromo-1-nitro-2-naphthol
[0041]
[0042] Add 66.6mg (0.3mmol) of 6-bromo-2-naphthol, 61.8mg (0.6mmol) of tert-butyl nitrite, 10.8mg (0.6mmol) of water, and tetrahydrofuran (2mL) into a dry 10mL schlenk bottle. Stir at room temperature for 2.5h. After the reaction was completed, the reaction solution was filtered through a glass dropper containing silica gel, rinsed with ethyl acetate, the filtrate was spin-dried, and separated by column chromatography to obtain the target product, a yellow solid, with a yield of 93%. 1 H NMR (300MHz, CDCl 3 ): 12.13(s, 1H), 8.77(d, J=9.3Hz, 1H), 7.94-7.87(m, 2H), 7.78-7.74(m, 1H), 7.27(t, J=9.0Hz, 1H) ; 13 C NMR (75MHz, CDCl 3 ): 158.8, 138.0, 133.9, 131.2(2), 129.9, 125.5, 125.1, 120.8, 119.5; IR (KBr, cm -1 ): 1538, 1361; LRMS (EI, 70eV) m / z (%): 269 (M+2, 21), 267 (M + , 22), 222(100), 115(97).
Embodiment 18
[0043] Example 18 Synthesis of 2-nitro-1-naphthol
[0044]
[0045] Add 43.2 mg (0.3 mmol) of 1-naphthol, 61.8 mg (0.6 mmol) of tert-butyl nitrite, 10.8 mg (0.6 mmol) of water, and tetrahydrofuran (2 mL) into a dry 10 mL schlenk bottle, and stir the reaction bottle at room temperature 4h. After the reaction was completed, the reaction solution was filtered through a glass dropper containing silica gel, rinsed with ethyl acetate, the filtrate was spin-dried, and separated by column chromatography to obtain the target product, a yellow solid, with a yield of 82%. 1 H NMR (300MHz, CDCl 3 ): 12.23(s, 1H), 8.49(d, J=8.1Hz, 1H), 7.98(d, J=9.6Hz, 1H), 7.80(d, J=7.8Hz, 1H), 7.71(t, J =7.5Hz, 1H), 7.61(t, J=7.5Hz, 1H), 7.32(d, J=9.3Hz, 1H); 13 C NMR (75MHz, CDCl 3 ): 155.7, 138.7, 137.4, 131.4, 127.9, 127.1, 126.5, 125.1, 120.2, 119.4; IR (KBr, cm -1 ): 1540, 1370; LRMS (EI, 70eV) m / z (%): 189 (M + , 100), 115(74), 89(34).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com