Neuropeptide and synthesis method and application thereof
A synthesis method and neuropeptide technology, applied in the field of neuropeptides and their synthesis, can solve problems such as body load, toxic injury, temporary cure, etc., and achieve the effect of facilitating degradation, changing activity and state, and improving social memory defects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1 The synthesis of polypeptide (TAT-PBD) of the present invention
[0044] The specific sequence of the polypeptide is: tyrosine-glycine-arginine-lysine-lysine-arginine-arginine-glutamine--arginine-arginine-arginine-histamine Acid-Serine-Threonine-Threonine-Arginine-Valine (YGRKKRRQRRRHSTTRV)
[0045] 1. Main raw materials: Cl resin, amino acid, DIEA (N,N-diisopropylethylamine), DIC (N,N-diisopropylcarbodiimide), HOBT (1-hydroxybenzotriazole) , DMF (dimethylformamide), Piperidine (piperidine), trifluoroacetic acid, ethanedithiol, phenol, thioanisole, methanol, and water.
[0046] 2. Synthesis idea:
[0047] Resin: Cl-Resin
[0048] Route: Solid phase Fmoc(9-fluorenylmethoxycarbonyl)
[0049] Condensation: DIC+HOBT
[0050] To protect: Piperidine
[0051] Instrument: Fully automatic synthesizer
[0052] 3. Specific steps:
[0053] a. Connect the -COOH of the first amino acid (V valine) to Cl-Resin with DIEA, and then block the unreacted functional grou...
Embodiment 2
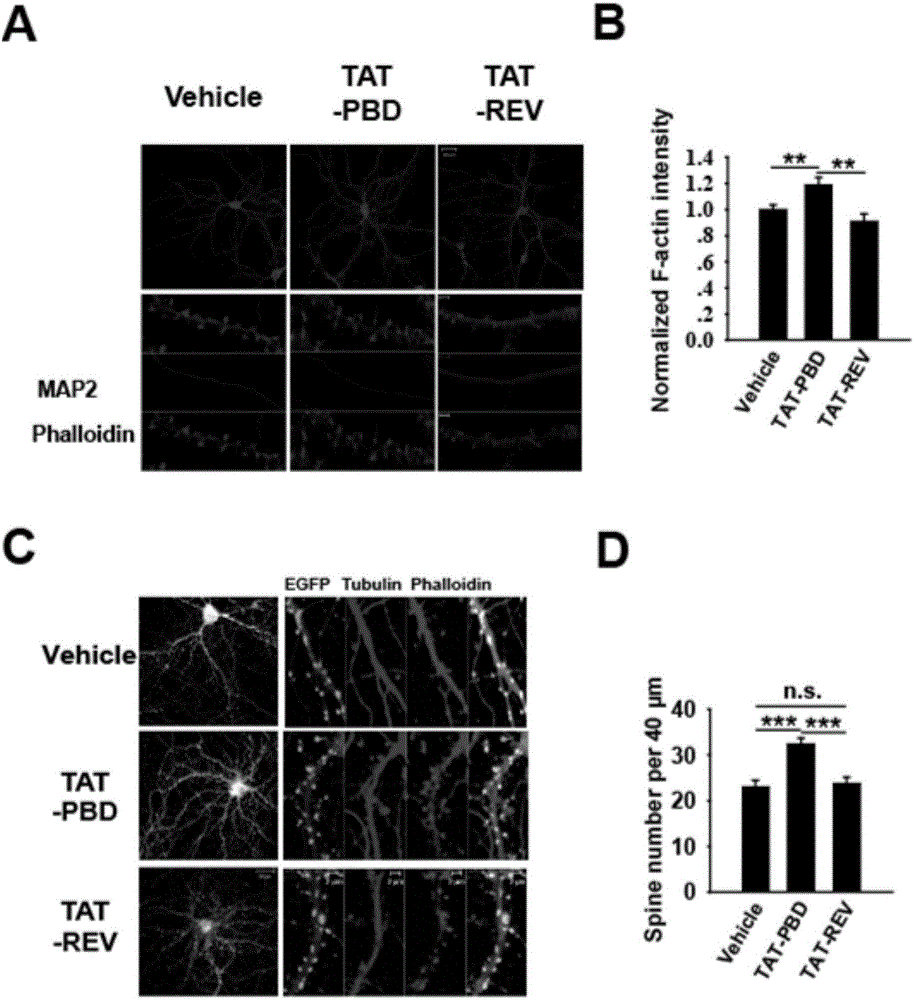
[0071] Add this product (TAT-PBD) and control polypeptide to the medium of mouse hippocampal neurons cultured in vitro for 20 days to a final concentration of 50 micrograms per milliliter, fix the cells with 4% paraformaldehyde solution one hour later, and carry out Immunostaining, as attached figure 1 As shown, it can be observed that the number of dendritic spines of neurons treated with TAT-PBD was significantly increased.
Embodiment 3
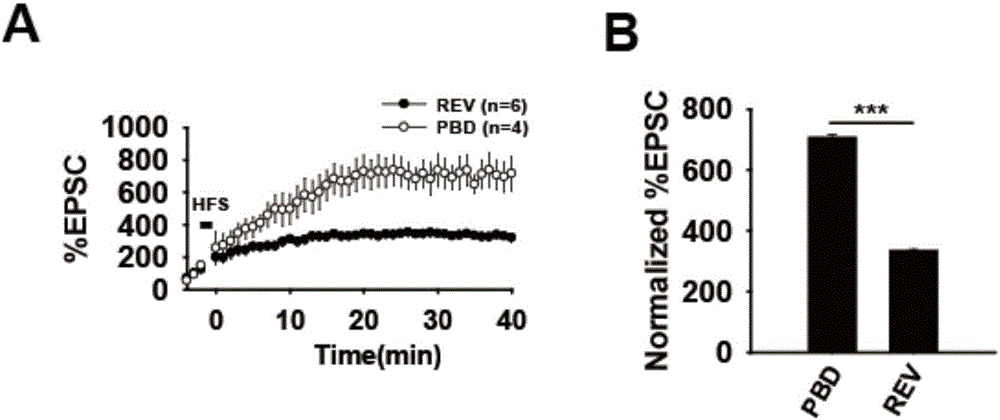
[0073] Acute brain slices of wild-type mice were prepared, and whole-cell electrophysiological recordings were performed in the CA3-CA1 loop of the hippocampus, and TAT-PBD polypeptide (50 mg / ml) was added to the intracellular fluid. The results show (as attached figure 2 shown), compared with the control group, PBD could significantly increase the amplitude of LTP.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com