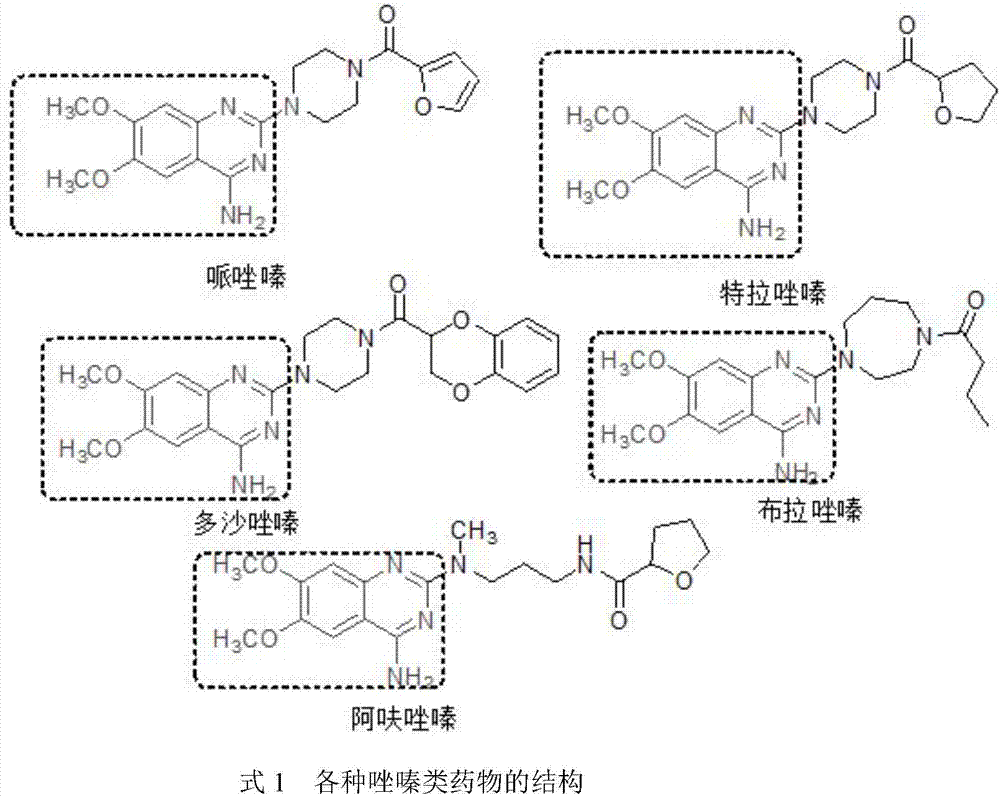
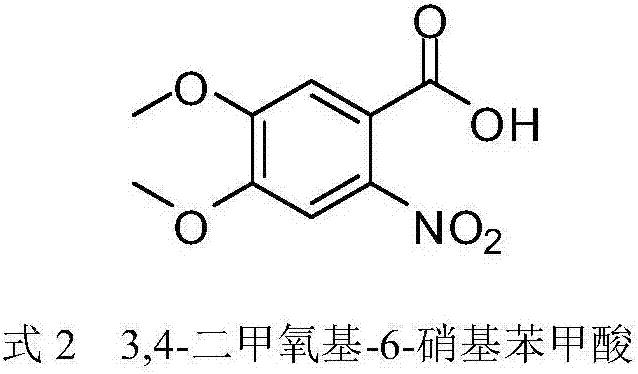
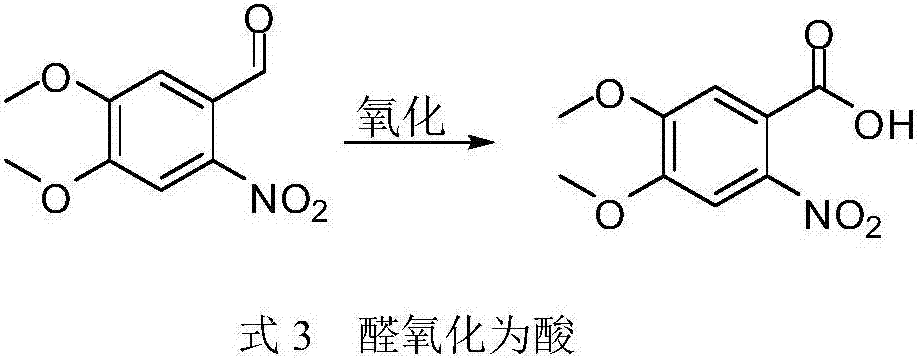
Synthesis method of 3,4-dimethoxy-6-nitrobenzoic acid
A technology of nitrobenzoic acid and dimethoxy, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of difficult control of reaction process, difficult industrialization, environmental pollution, etc. Inexpensive, simple preparation method and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] In 3,4-dimethoxy-6-nitrobenzaldehyde (10g), add reaction medium (water 20mL, methanol 30mL), add acetic acid (3.5mL), 30% hydrogen peroxide (9mL), stir well Add sodium chlorite (content 80%, 9g) and dissolve in water (5mL); stir and heat up to about 50°C, monitor the reaction, add sodium bisulfite (10.5g) to quench the reaction after the reaction is completed, and evaporate the solvent to dryness. The product was dissolved in sodium hydroxide (6g×10mL) solution, filtered to remove impurities, and the filtrate was added with sulfuric acid until the pH was 1 to precipitate the product, filtered, and dried. 10 g of the product was obtained (93% yield, 99.5% purity by HPLC). NMR 1 H NMR (600MHz, CDCl 3 )δ7.41(s,0H),7.28(s,0H),4.01(d,J=8.0Hz,6H). 13 C NMR (101MHz, CDCl3) δ171.40, 153.75, 148.71, 124.60, 121.63, 112.33, 110.34, 56.09, 56.03.
Embodiment 2
[0027] Water (20 mL) and methanol (20 mL) were added to 3,4-dimethoxy-6-nitrobenzaldehyde (10 g), followed by stirring. Then add sodium dihydrogen phosphate (7.5g), 50% hydrogen peroxide (9mL), then add sodium chlorite (content 80%, 9g) dissolved in water (5mL), stir and heat up to about 40°C for reaction, monitor Reaction, after the reaction is over, add sodium bisulfite (16g) to quench the reaction, evaporate the solvent to dryness, dissolve the product in potassium hydroxide (6g×10mL) solution, filter to remove impurities, add hydrochloric acid to the filtrate until the pH is 1 to precipitate the product, filter ,drying. 9.7 g of the product was obtained (90% yield, 99.3% HPLC purity). NMR 1 H NMR (600MHz, CDCl 3 )δ7.41(s,0H),7.28(s,0H),4.01(d,J=8.0Hz,6H). 13 C NMR (101MHz, CDCl3) δ171.40, 153.75, 148.71, 124.60, 121.63, 112.33, 110.34, 56.09, 56.03.
[0028] The inventors of the present invention found in the process of preparing 3,4-dimethoxy-6-nitrobenzoic acid from...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com