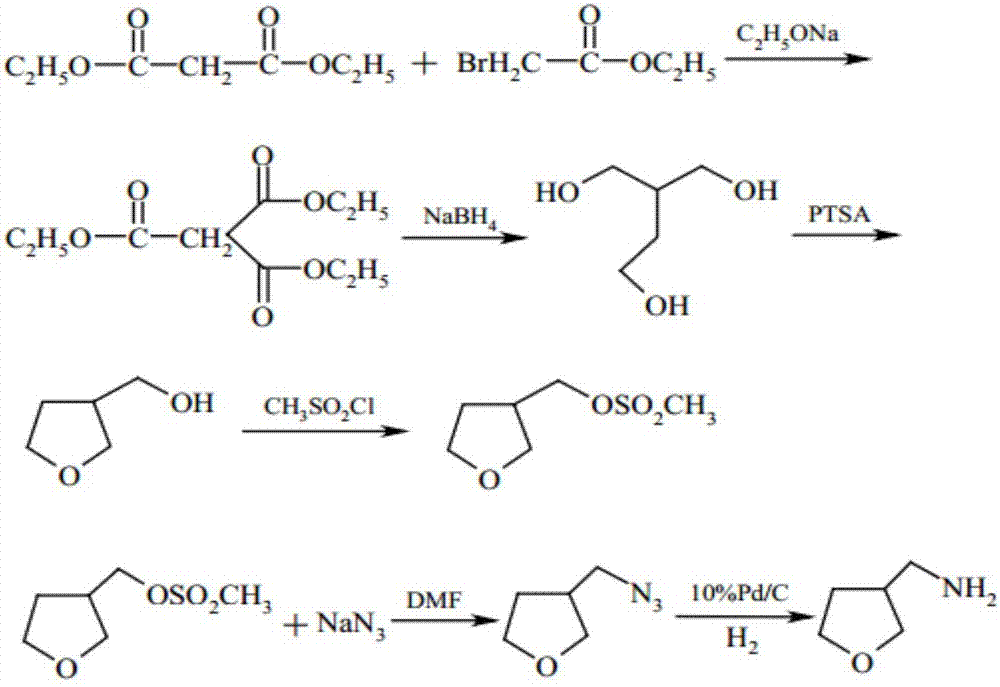
Synthetic method of 3-aminomethyl tetrahydrofuran
A technology of aminomethyltetrahydrofuran and a synthesis method, applied in the field of nicotinic insecticide dinotefuran, can solve the problems of unsuitability for industrial large-scale production, cumbersome reaction, long route and the like, and achieves strong safety and operability , The effect of simple process and high overall yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
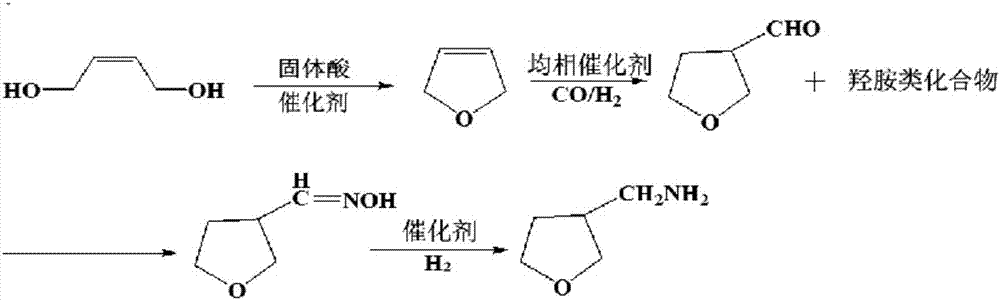
[0032] like figure 2 As shown, this embodiment includes the following steps:
[0033] Step 1. In a three-neck flask with a volume of 2L, add 1320g (15mol) of 1,4-butenediol, then add 1.32g of phosphotungstic heteropoly acid solid, and react at 250°C for 8h to obtain 2,5-dihydrofuran 1000g (14.3mol), the yield of the 2,5-dihydrofuran is 95.2%;
[0034] Step 2. Mix 1000g (14.3mol) of 2,5-dihydrofuran prepared in step 1 with 2L of methanol in an autoclave with a volume of 5L, and then add 20g of homogeneous catalyst HRhCO[P(Ph) 3 ] 3 , feed reaction gas into the autoclave to raise the pressure in the autoclave to 2 MPa, stir for 10 h at a temperature of 90° C., then lower the temperature, exhaust the gas, release the reaction product liquid, and distill off the methanol. Distillation obtains 3-formaldehyde tetrahydrofuran 1315g, and the yield of described 3-formaldehyde tetrahydrofuran is 92%; Described reaction gas is made of CO and H 2 Mixed according to the volume ratio o...
Embodiment 2
[0044] like figure 2 As shown, this embodiment includes the following steps:
[0045] Step 1. In a three-neck flask with a volume of 2L, add 1320g (15mol) of 1,4-butenediol, add 2g of ZSM-5 molecular sieve, and react at 230°C for 7h to obtain 1018g (14.5mol) of 2,5-dihydrofuran ), the yield of the 2,5-dihydrofuran is 97%;
[0046] Step 2. Mix 1018g (14.5mol) of 2,5-dihydrofuran prepared in Step 1 with 2L of ethanol in an autoclave with a volume of 5L, and then add 0.509g of homogeneous catalyst HRhCO[P(PhF) 3 ] 3 , feed reaction gas into the autoclave to raise the pressure in the autoclave to 4MPa, stir at 90°C for 18 hours, then lower the temperature, exhaust, release the reaction product liquid, and distill off ethanol, Distillation obtains 3-formaldehyde tetrahydrofuran 1396g, and the yield of described 3-formaldehyde tetrahydrofuran is 96%; Described reaction gas is made of CO and H 2 Mixed according to the volume ratio of 1:1;
[0047] Step three, mix 1396g (13.9mol...
Embodiment 3
[0051] like figure 2 As shown, this embodiment includes the following steps:
[0052] Step 1. In a three-neck flask with a volume of 2L, add 1320g (15mol) of 1,4-butenediol, add 4.4g of phosphomolybdoheteropolyacid solid, and react at 230°C for 5h to obtain 966g of 2,5-dihydrofuran (13.8mol), the yield of the 2,5-dihydrofuran is 92%;
[0053] Step 2. Mix 966g (13.8mol) of 2,5-dihydrofuran prepared in step 1 with 2.5L methanol in an autoclave with a volume of 5L, and then add 19.3g of homogeneous catalyst HRhCO[P(PhSO 3 Na) 3 ] 3 , feed reaction gas into the autoclave to raise the pressure inside the autoclave to 7MPa, stir for 15 hours at a temperature of 60°C, then lower the temperature, exhaust the gas, release the reaction product liquid, and distill off the methanol. Distillation obtains 3-formaldehyde tetrahydrofuran 1311g, and the yield of described 3-formaldehyde tetrahydrofuran is 95%; Described reaction gas is made of CO and H 2 Mixed according to the volume rat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com