Small-molecule fluorescent probe used for ratio recognition of human carbonic anhydrase, and synthetic method and application thereof
A technology of carbonic anhydrase and fluorescent probes, which can be used in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., and can solve urgent needs and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
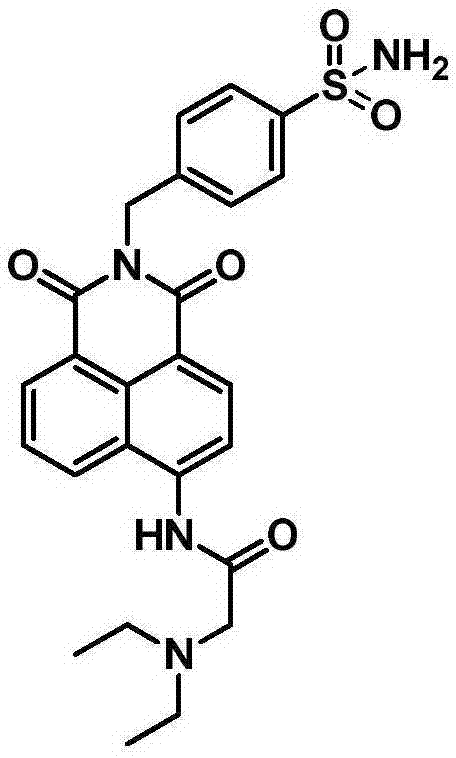
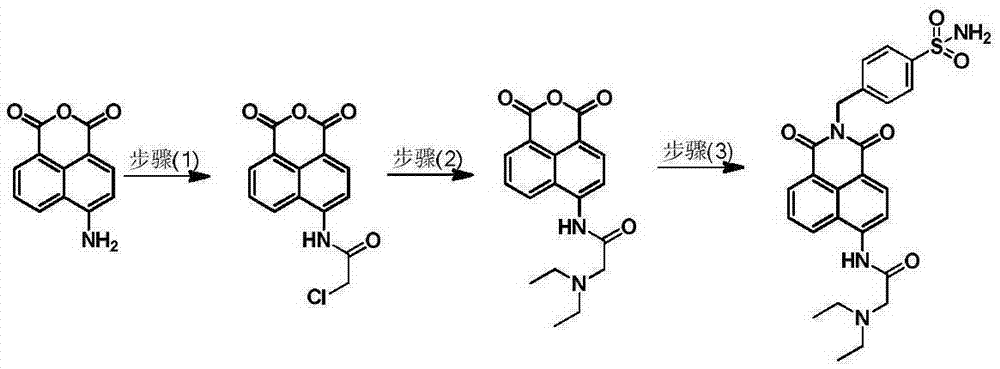
[0032] Example 1: Synthesis of small molecule fluorescent probes for ratiometric recognition of human carbonic anhydrase.
[0033] (1) Synthesis of intermediate 4-(2-chloroacetyl)amino-1,8-naphthalene anhydride:
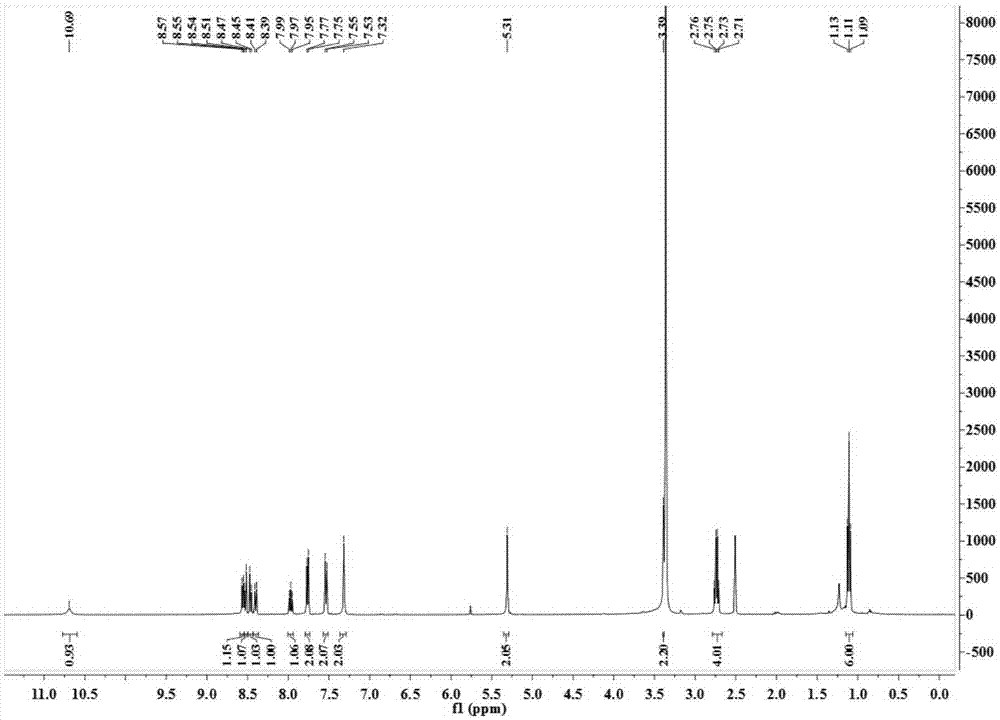
[0034] 4-Amino-1,8-naphthalene anhydride (1.0 g, 4.7 mmol) was placed in 60 mL of tetrahydrofuran, and 0.5 mL of chloroacetyl chloride was added under ice-cooling. Stir at room temperature overnight (14h), remove the solvent under reduced pressure, separate on a silica gel column with dichloromethane as eluent, and remove the solvent under reduced pressure to obtain 1.0 g of off-white solid with a yield of 76%. 1 HNMR (400MHz, DMSO) δ10.86(s, 1H), 8.80(d, J=8.8Hz, 1H), 8.59-8.63(m, 2H), 8.37(d, J=8.0Hz, 1H), 8.02( t,J=7.8Hz,1H), 4.62(s,2H).
[0035]
[0036] (2) Synthesis of intermediate 4-(2-(N,N-diethylamino)acetyl)amino-1,8-naphthalene anhydride:
[0037] 4-(2-Chloroacetyl)amino-1,8-naphthalene anhydride (500 mg, 1.7 mmol) was placed in 50 mL of acetonitrile...
Embodiment 2
[0043] Example 2: The response of the fluorescent probe prepared in Example 1 to different solvents.
[0044] Figure 5 The concentration of the fluorescent probe prepared in Example 1 is 10 μM, and the excitation light is 370nm. In 20mM pH=7.4PBS buffer solution, the probe exhibits a blue fluorescence with a wavelength of ~470nm. In methanol, dimethyl sulfoxide, N, In N-dimethylformamide, acetonitrile, and ethanol, the main fluorescence peak red-shifted to 525-560nm; indicating that the fluorescent probe can exhibit long-wave properties in a hydrophobic environment.
Embodiment 3
[0045] Example 3: The fluorescent probe prepared in Example 1 responds to 1 equivalent of carbonic anhydrase within a sustained period of time.
[0046] Image 6 The concentration of the fluorescent probe prepared in Example 1 in 20mM pH=7.4PBS buffer solution was 1 μm, and the excitation light was 405nm. After adding 1 equivalent of carbonic anhydride 1 of the fluorescent probe, the fluorescence intensity at a wavelength of ~470nm was significantly reduced, while the wavelength The fluorescence intensity at ~530nm increased sharply, and there was no significant change within 48 hours; it indicated that the fluorescent probe entered the hydrophobic cavity of carbonic anhydrase 1, and the fluorescence showed long-wave properties. .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com