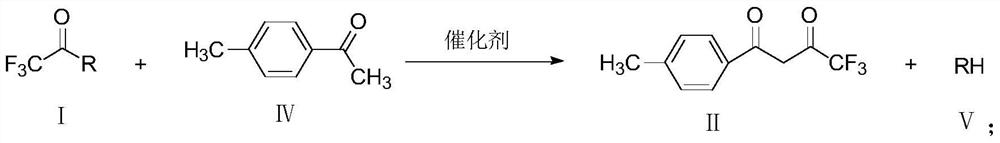
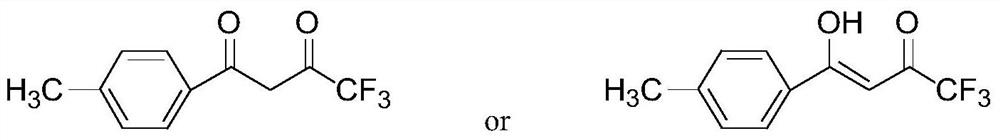A method for preparing 1-p-methylphenyl-4,4,4-trifluoro-1,3-butanedione
A technology of p-methylphenyl and p-methylacetophenone, which is applied in the field of fluorine compound preparation, can solve the problems of long reaction time, environmental pollution, and complicated acidification steps in the post-treatment process, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
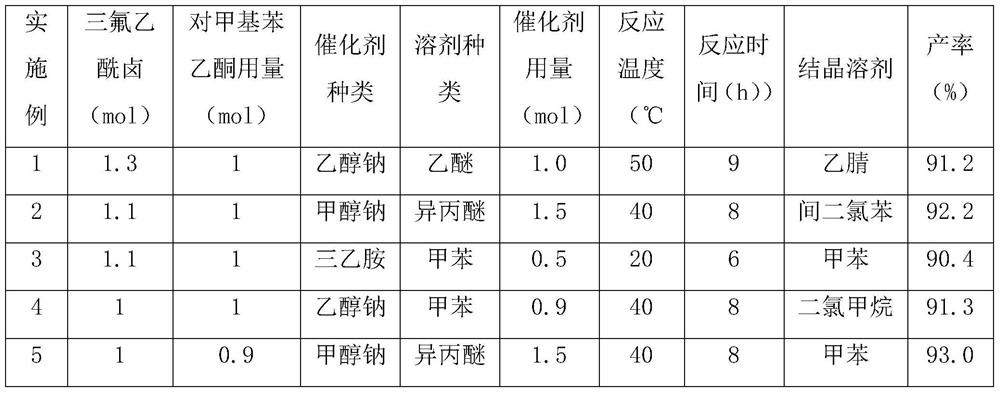
[0028] In a 1000 mL three-necked flask, add 500 mL of ether, add 68.0 g (1.0 mol) of sodium ethoxide and 134 g (1.0 mol) of p-methylacetophenone under stirring, heat the reaction to 40 ° C, and after 6 hours of reaction, distill off the solvent. A yellow solid was obtained, which was partially dissolved by adding 300ml of acetonitrile, and 230.1g (1.3mol) of trifluoroacetyl bromide was introduced into the reaction solution, reacted at 50°C for 3h, and the filtrate was obtained by suction filtration, part of the acetonitrile was distilled off, and 210g of a white solid was obtained at -10°C , the content is 99.2%, and the yield is 91.2%.
Embodiment 2
[0030] In a 1000mL three-necked flask, add 500mL of isopropyl ether, add 81g (1.5mol) of sodium methylate and 134g (1.0mol) of p-methylacetophenone under stirring, and heat the reaction to 30°C. After 6 hours of reaction, evaporate the solvent , add 250ml of m-dichlorobenzene to partially dissolve, pass 127.6g (1.1mol) trifluoroacetyl fluoride into the reaction solution, react at 40°C for 2h, obtain the filtrate by suction filtration, distill off part of m-dichlorobenzene, and obtain white The solid is 212g, the content is 99.0%, and the yield is 92.2%.
Embodiment 3
[0032] In a 1000mL three-necked flask, add 500mL of toluene, add 50.5g (0.5mol) of triethylamine and 134g (1mol) of p-methylacetophenone under stirring, and pass 135.3g (1.1mol) of trifluoroacetyl chloride into the reaction solution , reacted at 20°C for 6h, obtained the filtrate by suction filtration, evaporated part of the toluene, and obtained 208g of white solid at -10°C, the content was 99.2%, and the yield was 90.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com