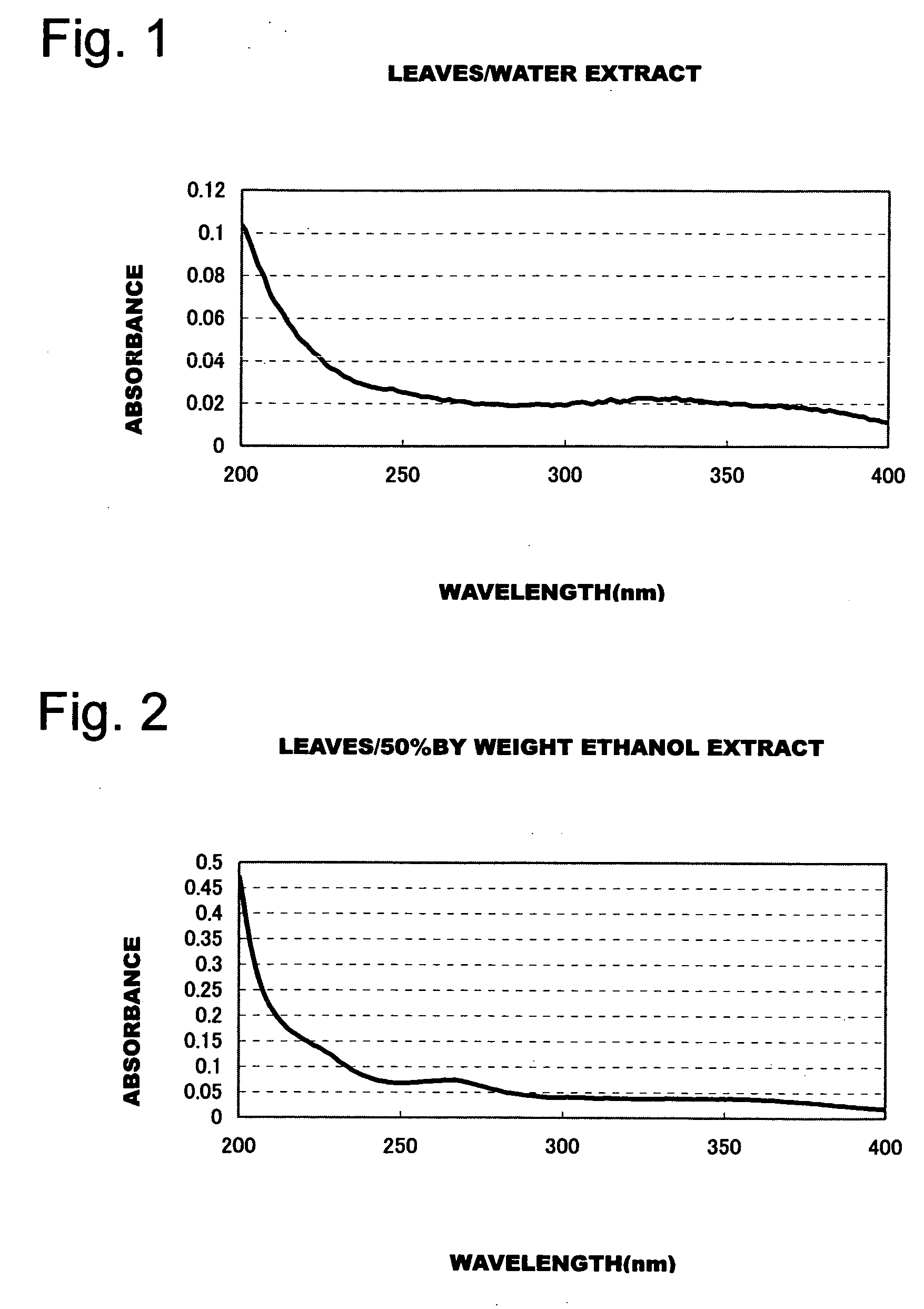
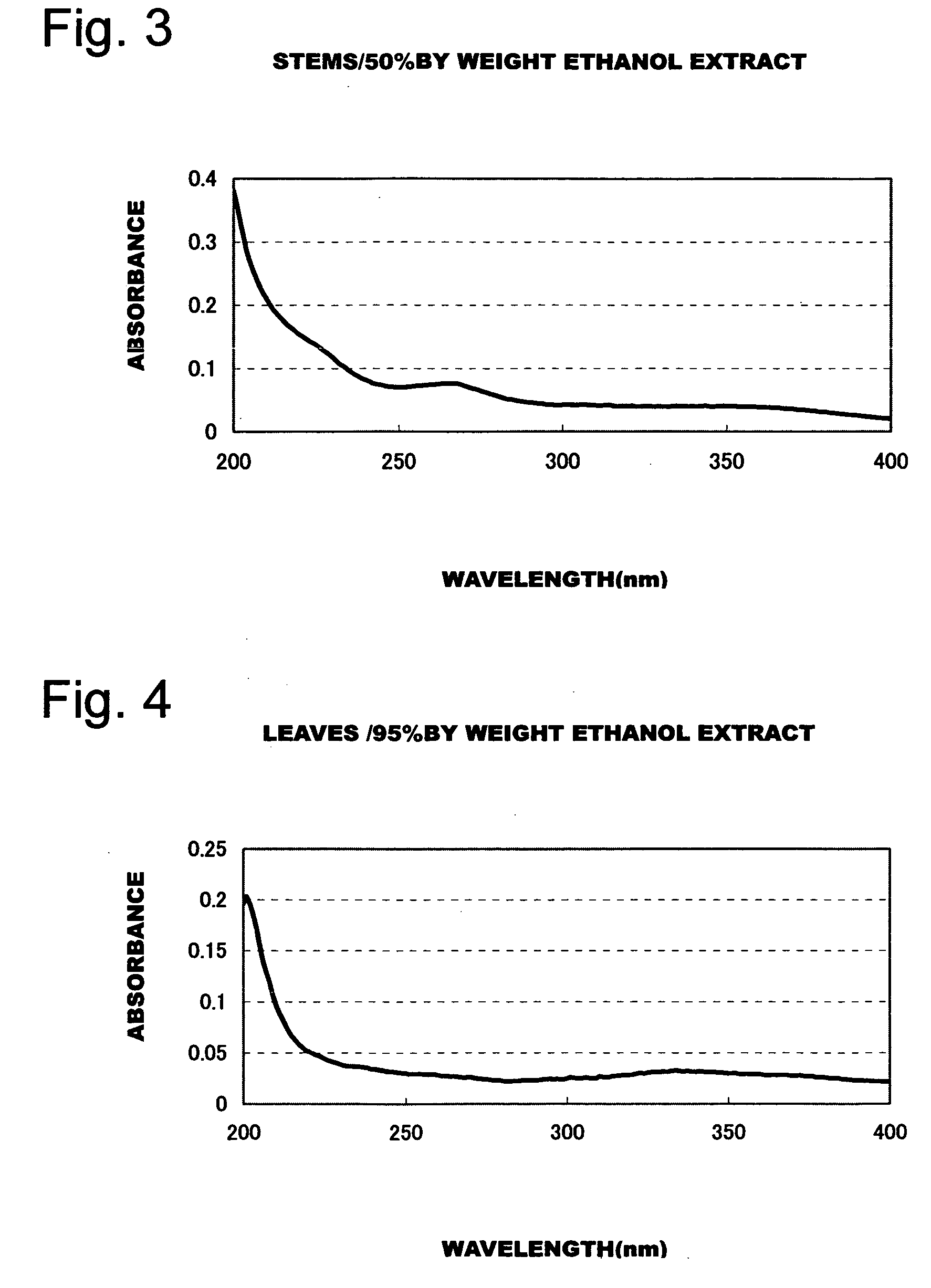
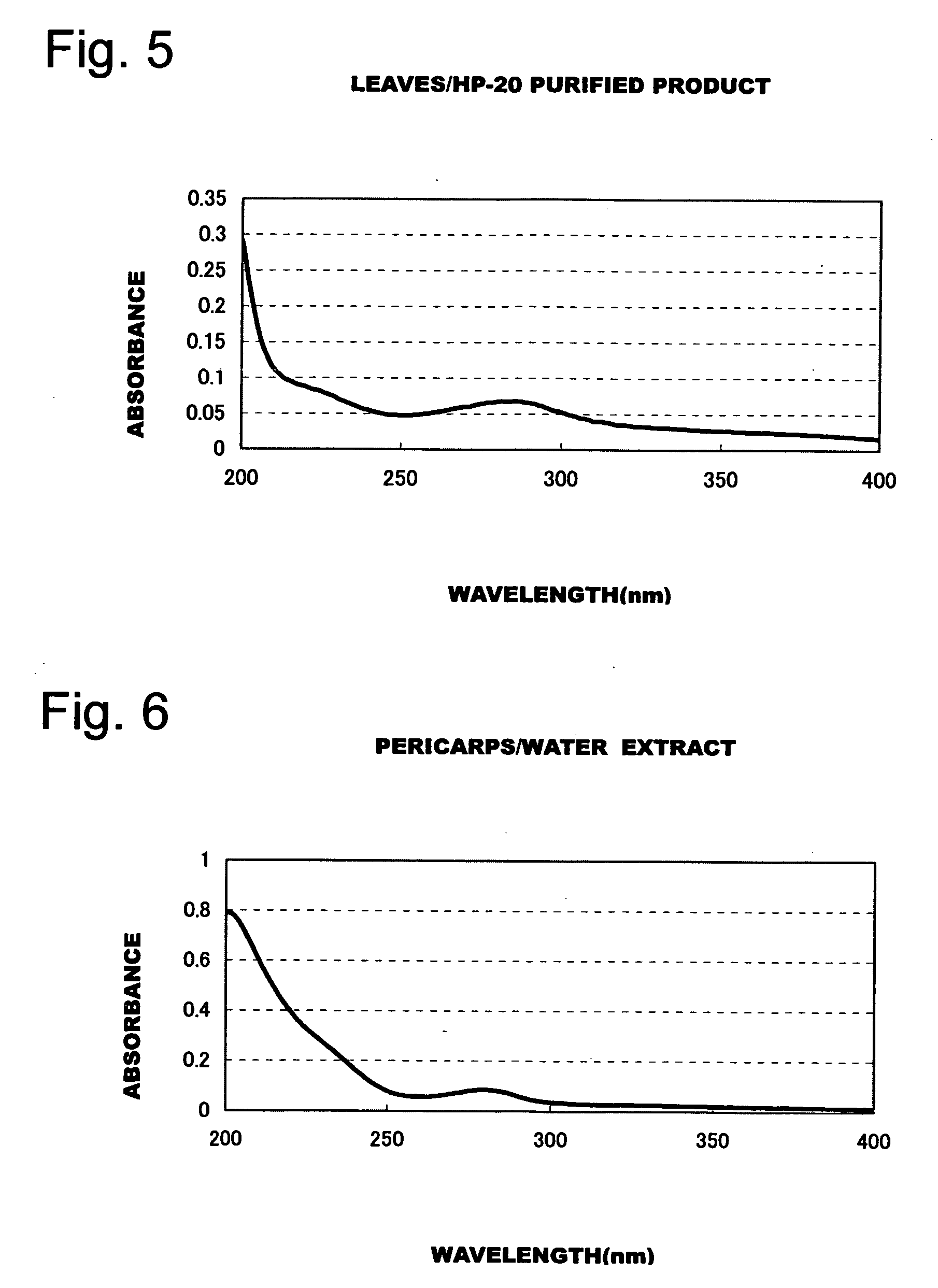
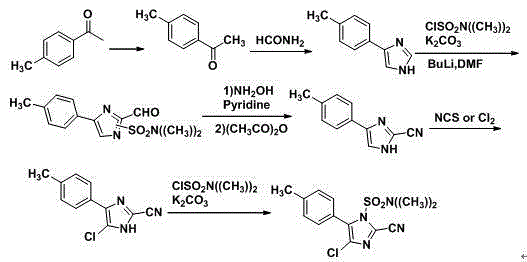
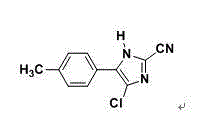
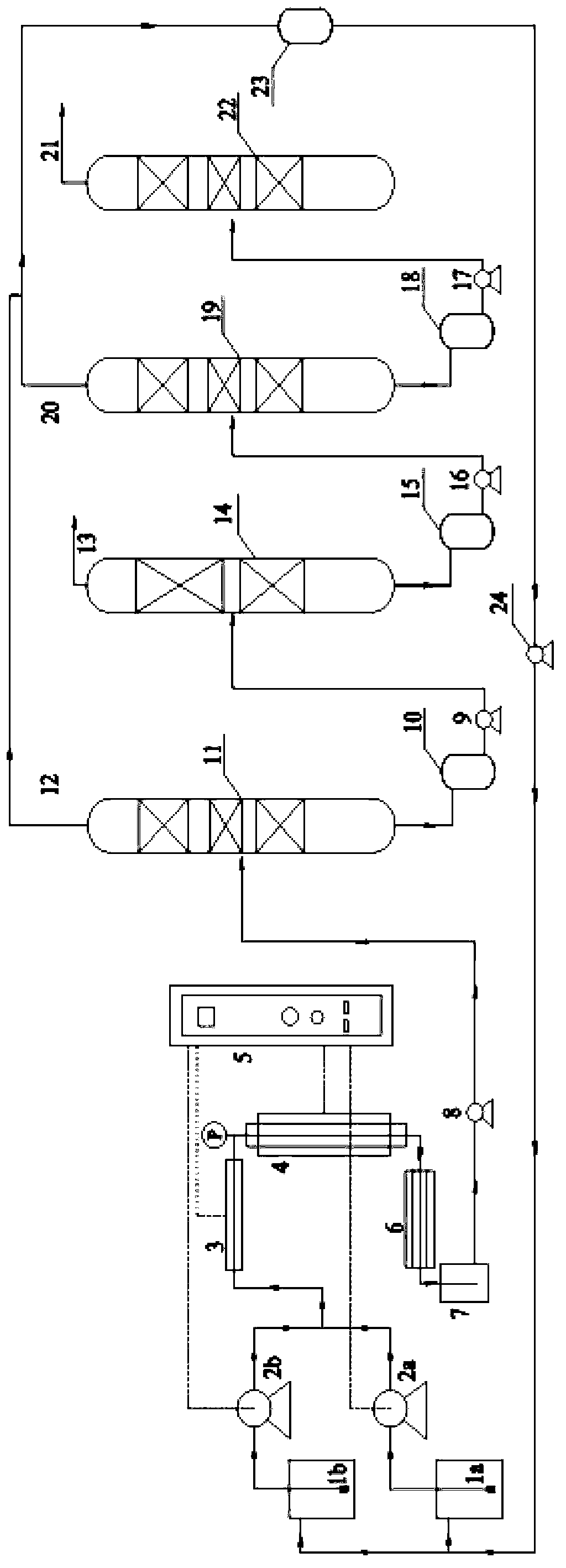
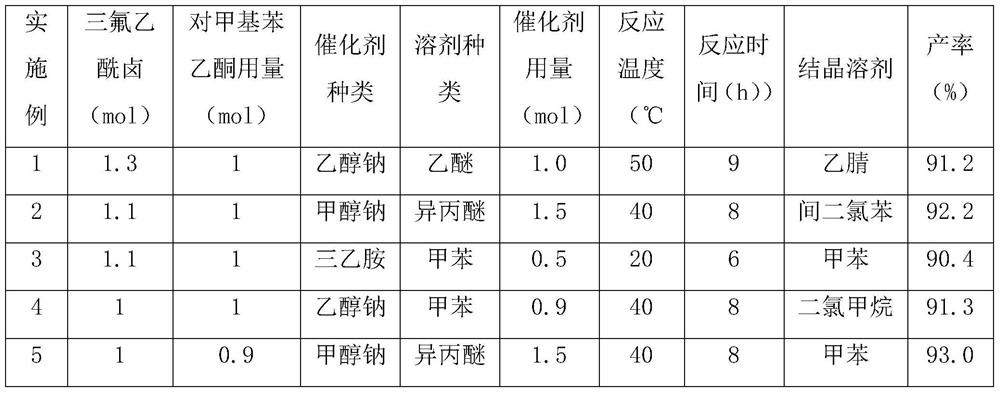
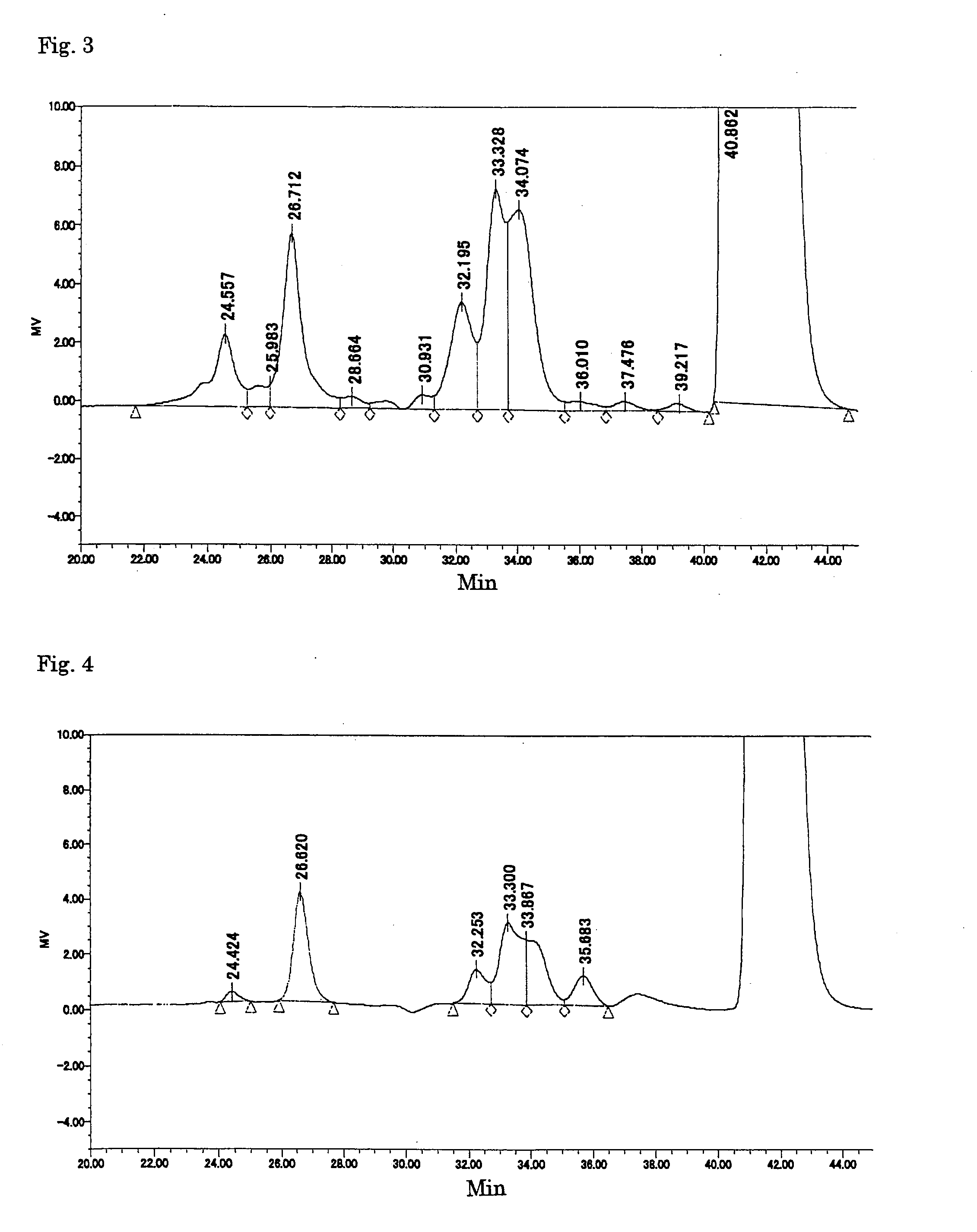
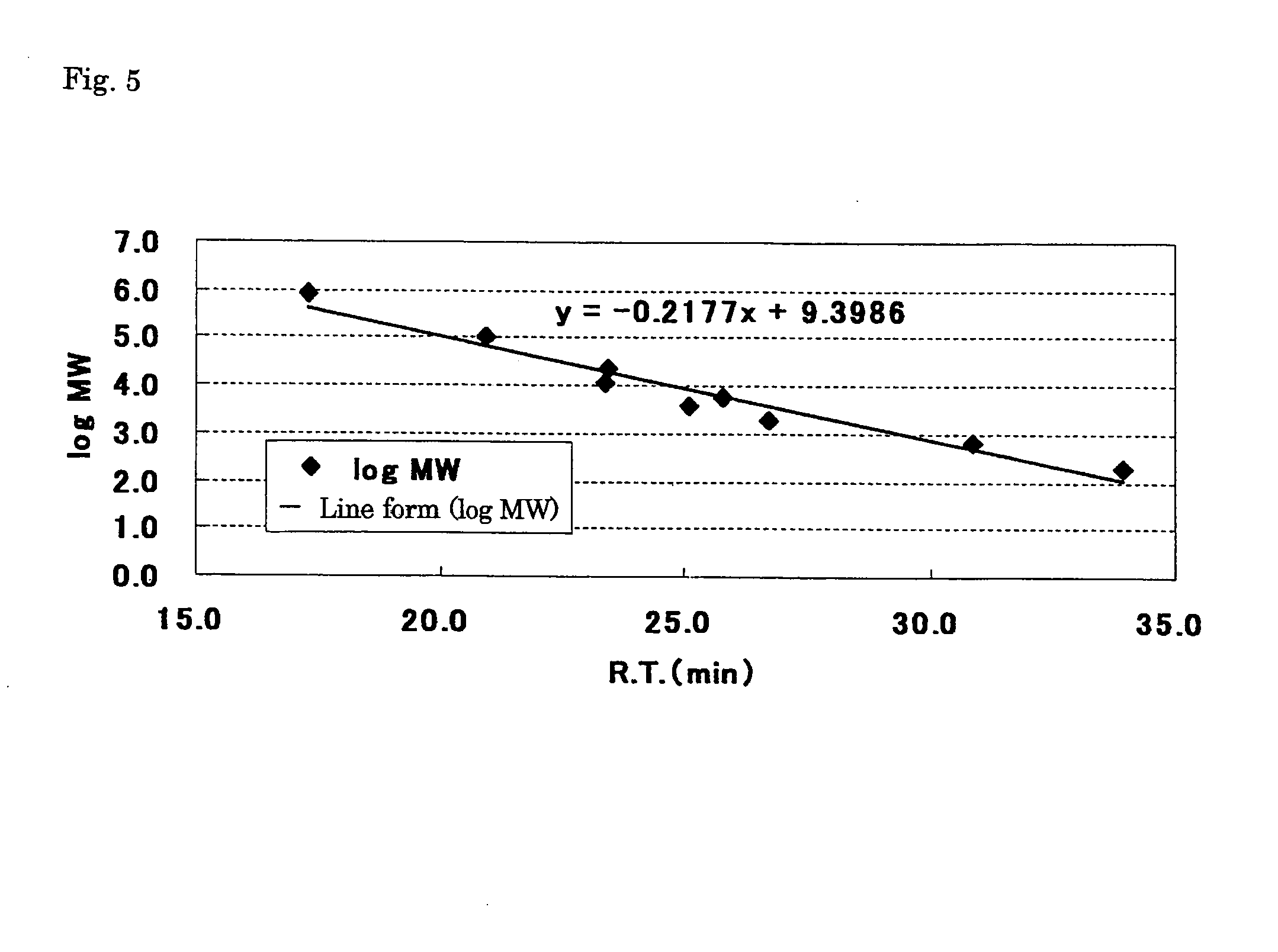
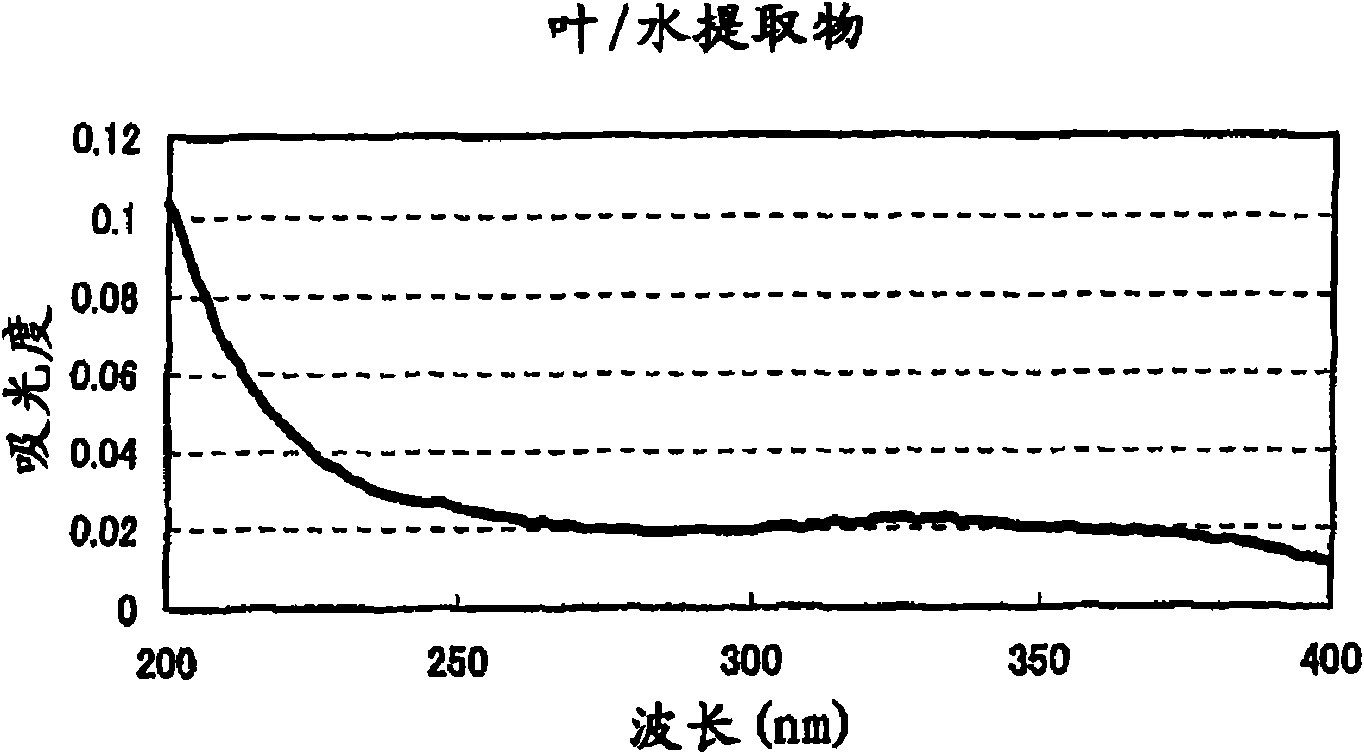
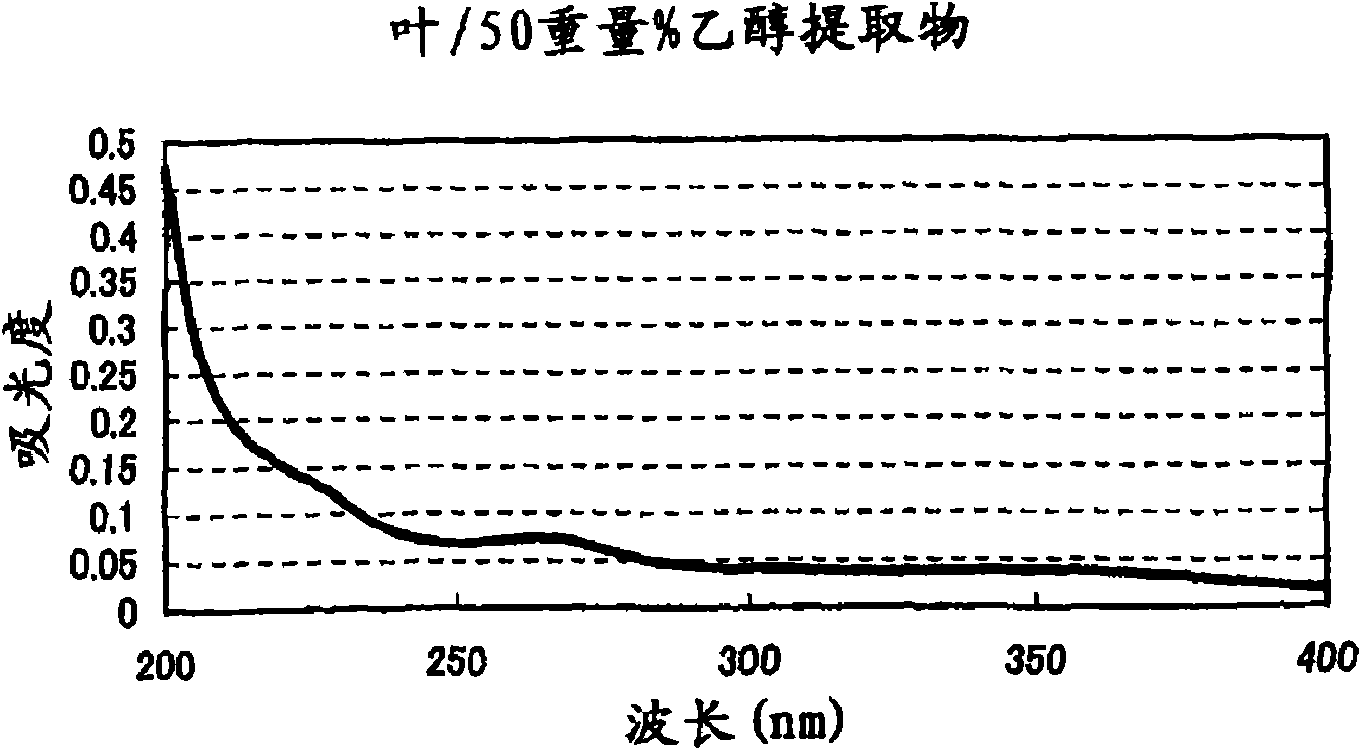
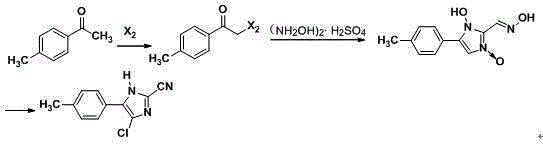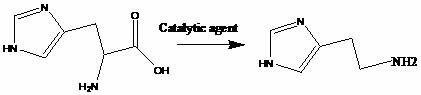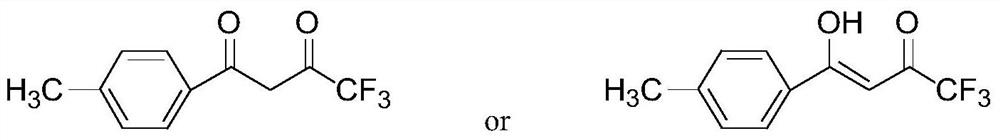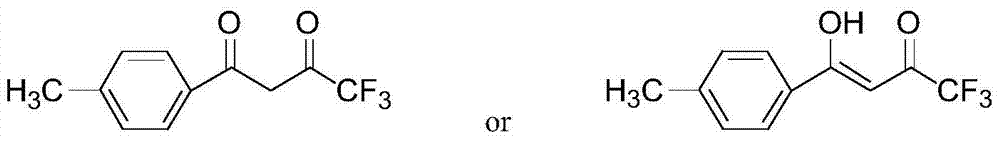Patents
Literature
36 results about "P-methylacetophenone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
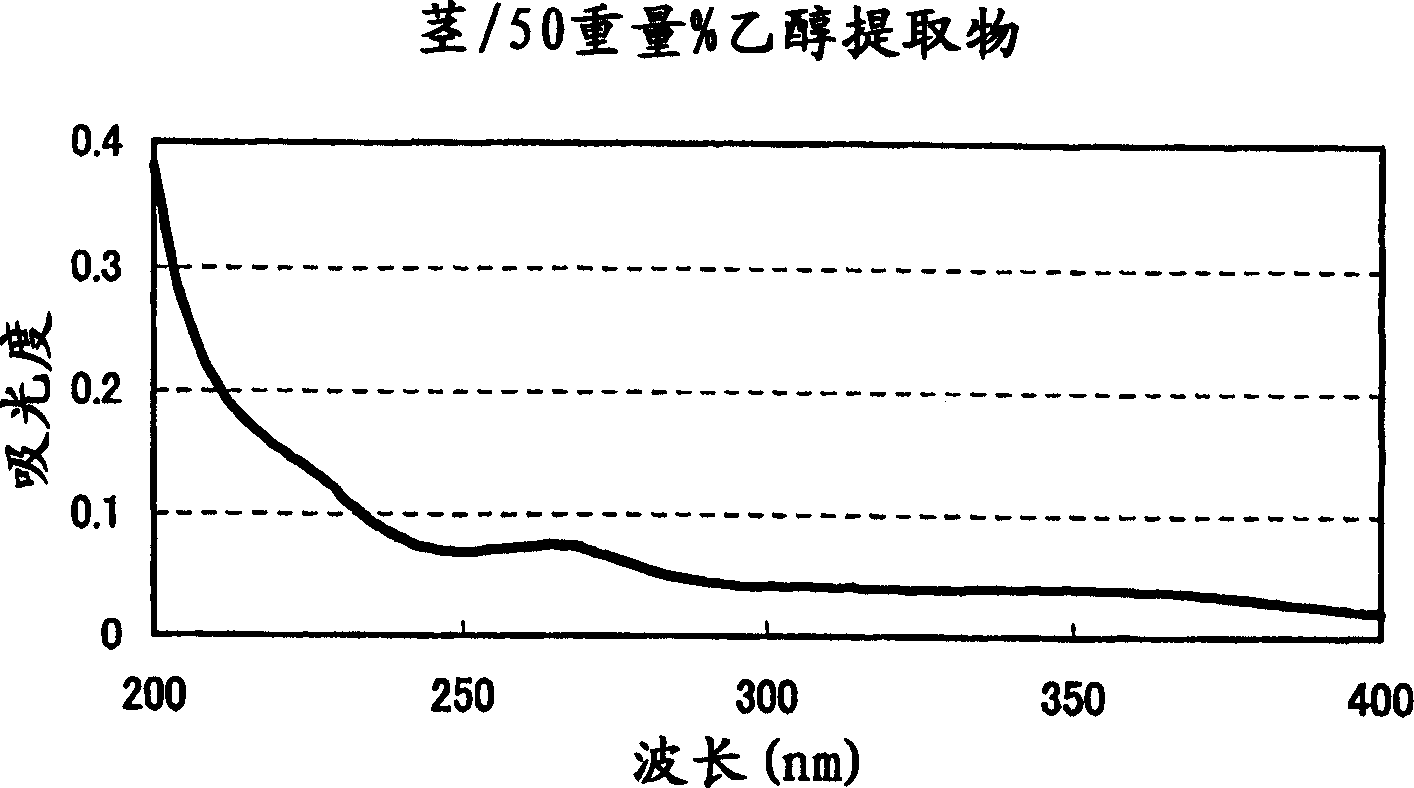
4-Methyl Acetophenone is a natural-identical substance with aromatic, sweet, vanilla, and cumin olfactory notes as well as sweet, citrus, fruity, and cherry tastes. It is a colorless, pale yellow liquid that is insoluble in water but soluble in organic solvents.
Method for synthesizing loxoprofen sodium
ActiveCN101412670ARaw materials are easy to getUnique craftOrganic compound preparationCarboxylic compound preparationSolventHydrolysis
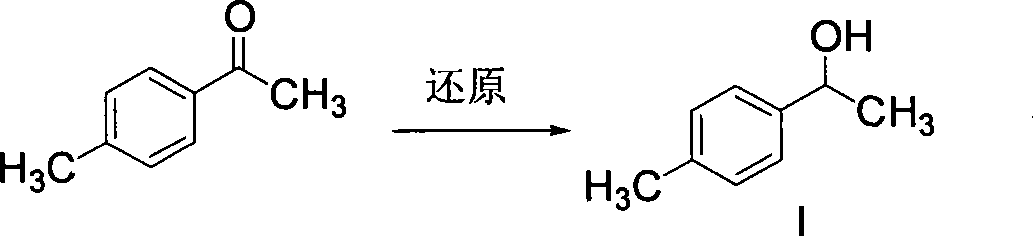
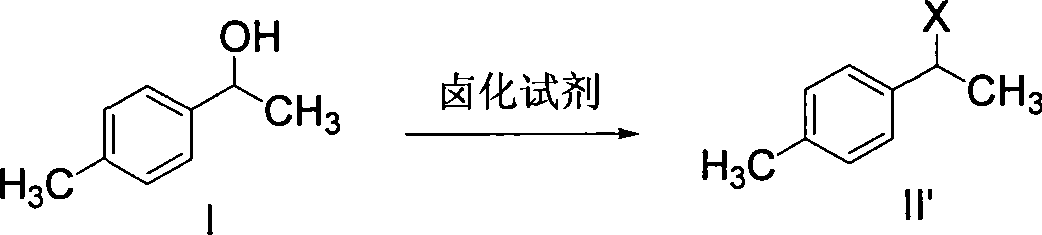
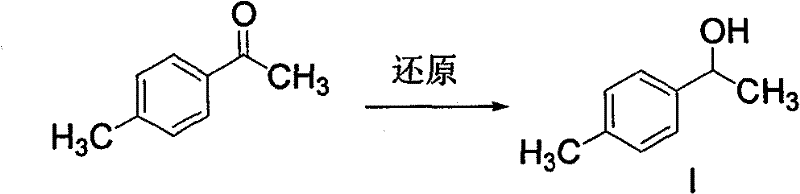
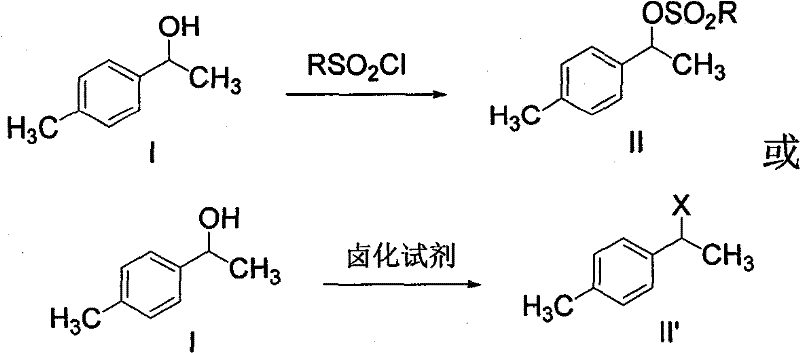
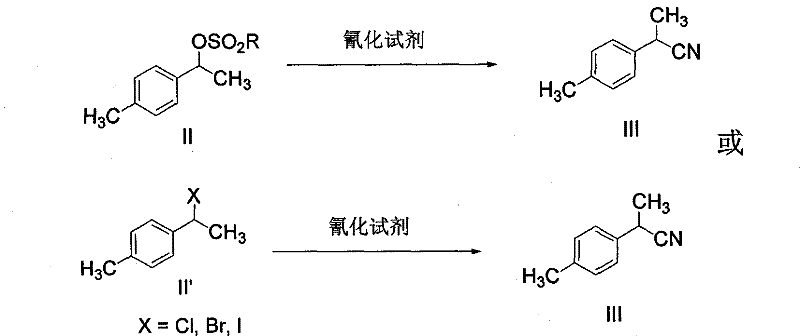
The invention discloses a synthetic method for loxoprofen sodium, which is prepared by taking methyl acetophenone as an initial raw material through reduction, acylation or halogen substituent, cyanation, hydrolysis, bromination, condensation, decarboxylation and salifying. The method has the advantages of easily obtained raw material, unique technology, simple and stable operation, and high productive rate in each step of reaction; and all solvents used in the synthesis process can be recycled, so the production cost is reduced greatly. Tests show that the obtained product has reliable quality and stable performance, and can be further used for making preparation of non-steroidal anti-inflammatory drugs such as the loxoprofen sodium.
Owner:ZHEJIANG APELOA JIAYUAN PHARMA +1
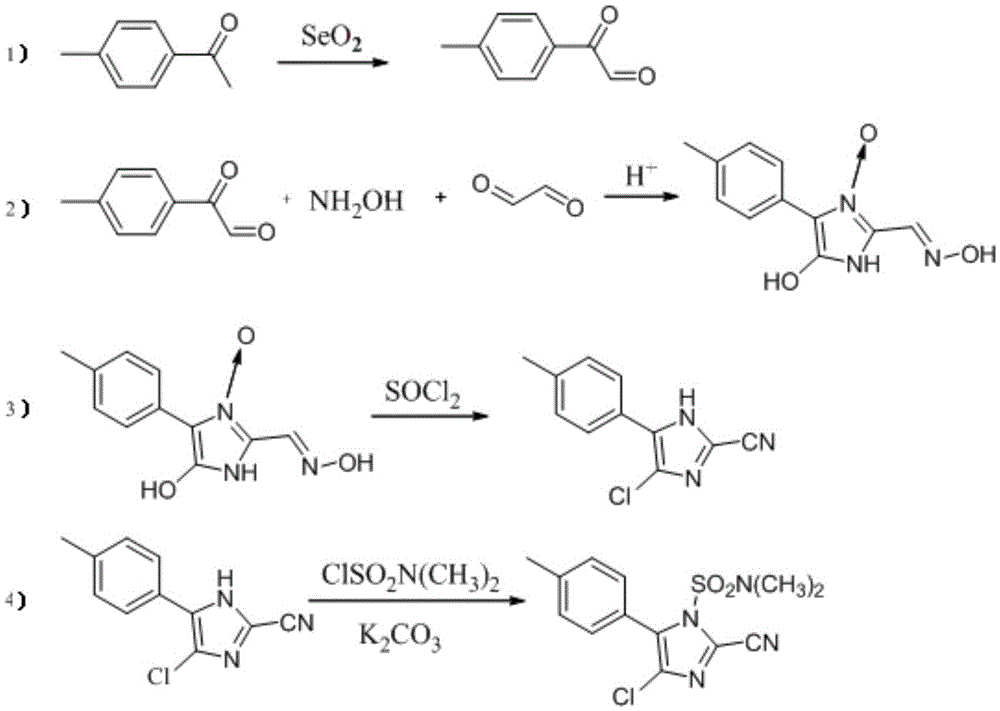
Synthesis method of 4-chloro-2-cyano-N,N-dimethyl-5-(4-methylphenyl)-1H-imidazole-1-sulfonamide
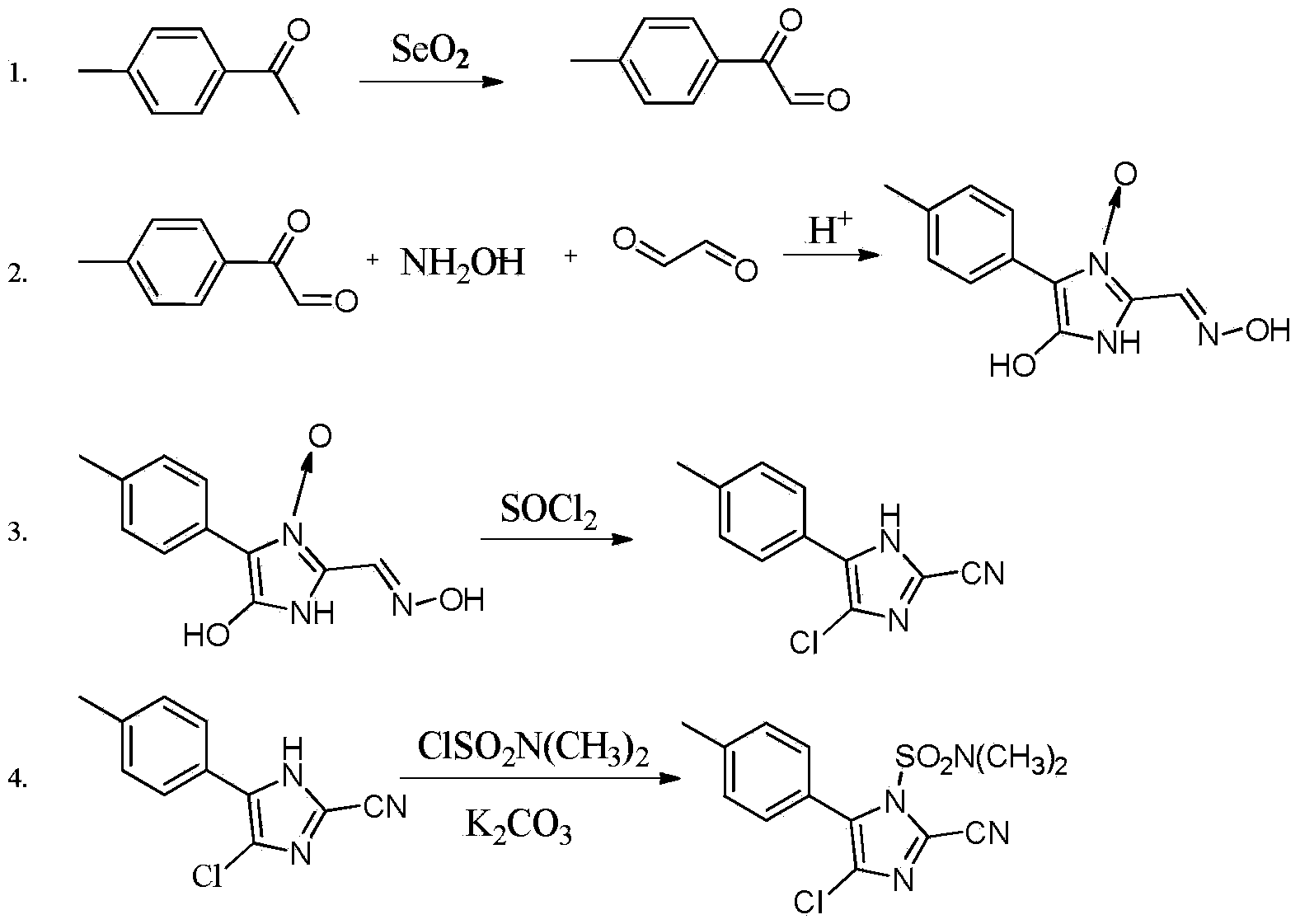
The invention discloses a synthesis method of 4-chloro-2-cyano-N,N-dimethyl-5-(4-methylphenyl)-1H-imidazole-1-sulfonamide (cyazofamid). According to the synthesis method, methylacetophenone is taken as a raw material, selenium dioxide is used for oxidation to prepare an intermediate, namely 2-carbonyl-2-p-benzyl aldehyde, then N,N-dimethylformamide and the like are taken as solvents, thionyl chloride is taken as a chlorinating agent and reducing agent for preparing the intermediate, and then intermediate further reacts with N,N-dimethyl sulfonamide chloride to synthesize the cyazofamid. Compared with the prior art, the synthesis method has the advantages that the reaction period is shortened, the post-treatment process is simplified and the synthesis method is suitable for mass industrial production.
Owner:XIAN MODERN CHEM RES INST
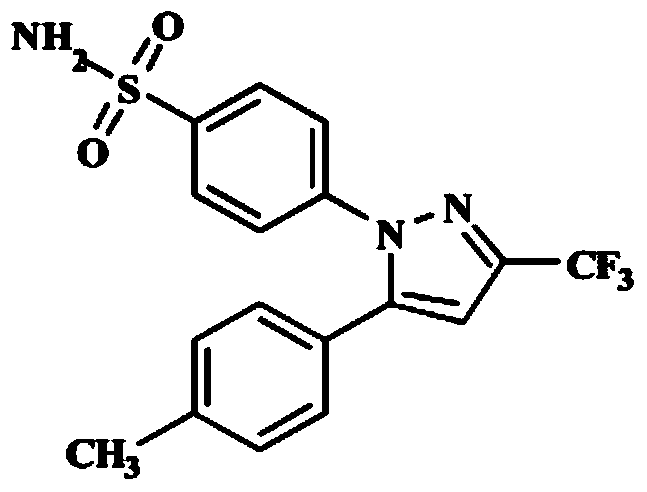
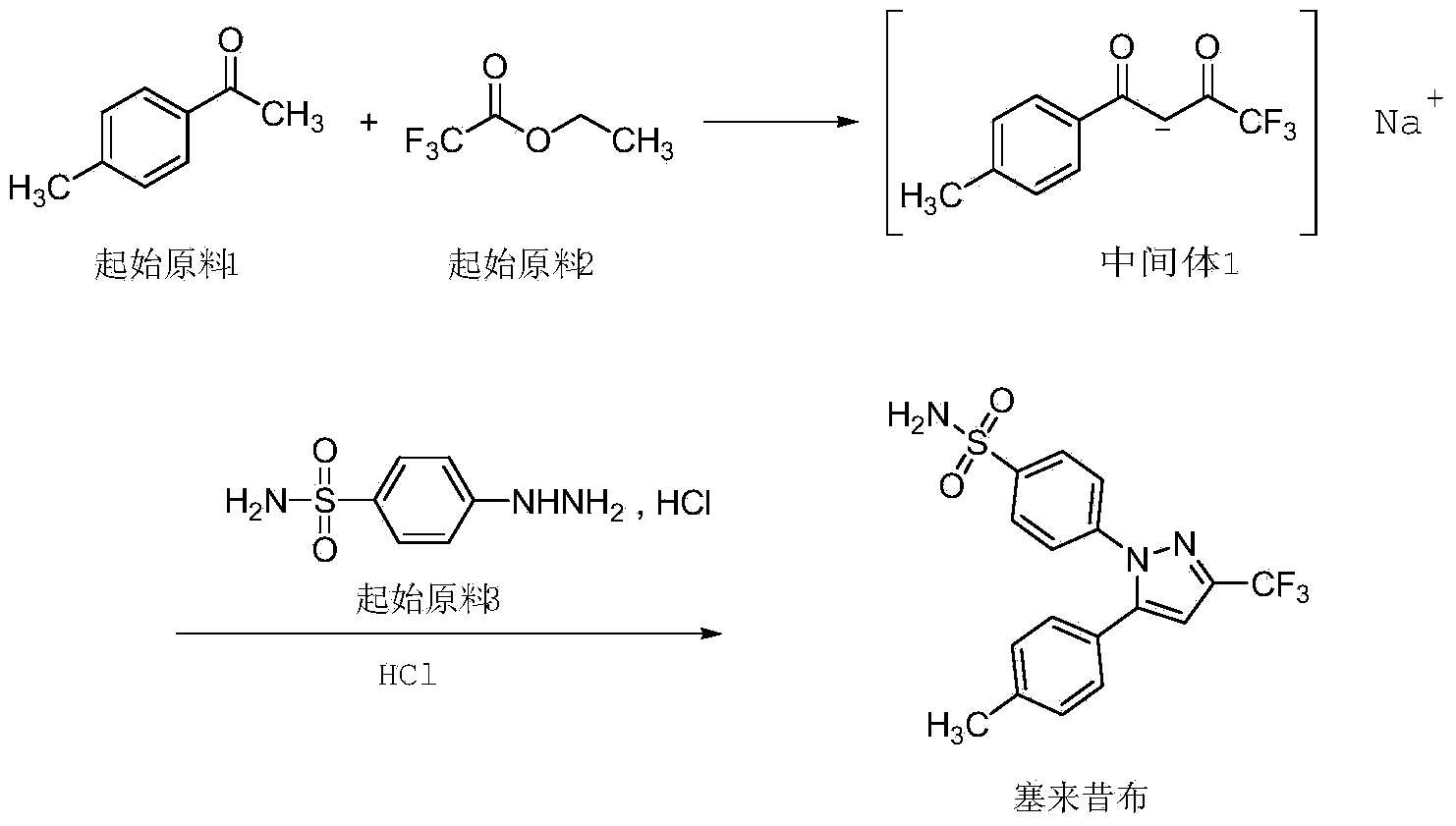
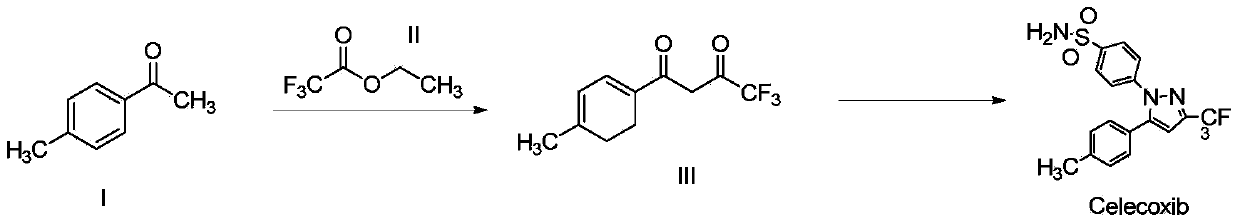
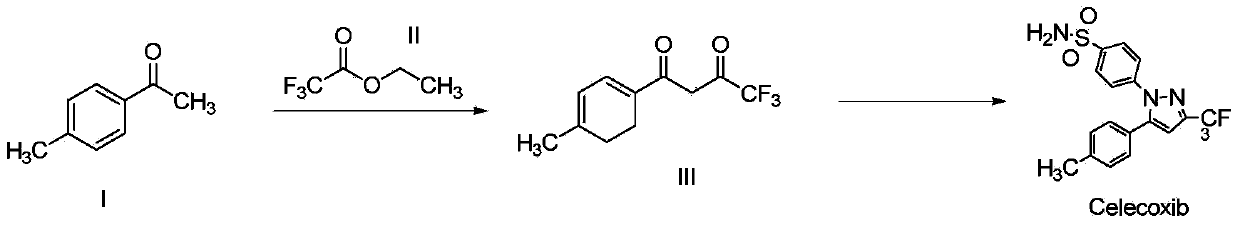
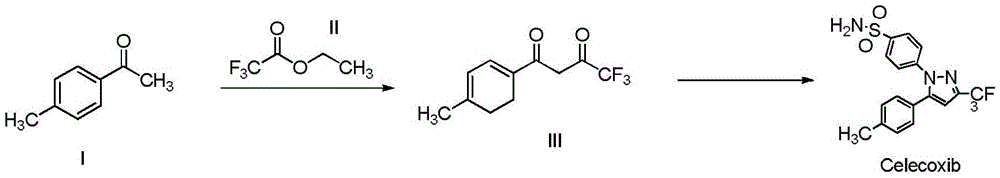
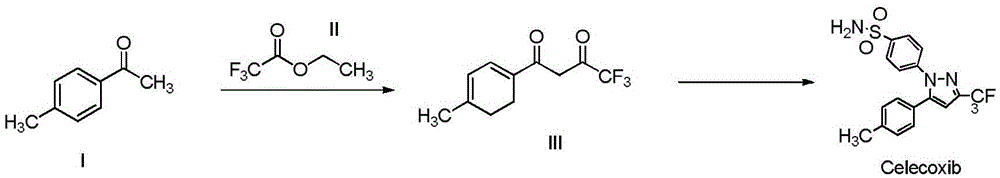
Synthesis method of celecoxib
InactiveCN102391184AHigh purityHigh yieldOrganic chemistryPhenylhydrazine hydrochlorideClaisen condensation
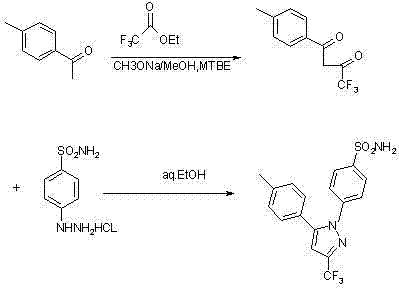
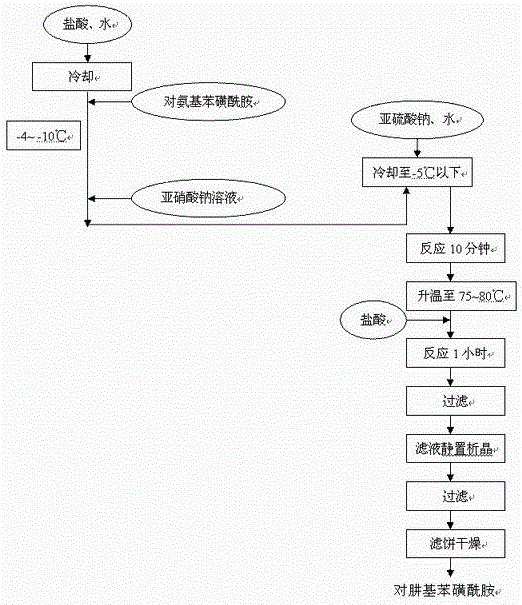
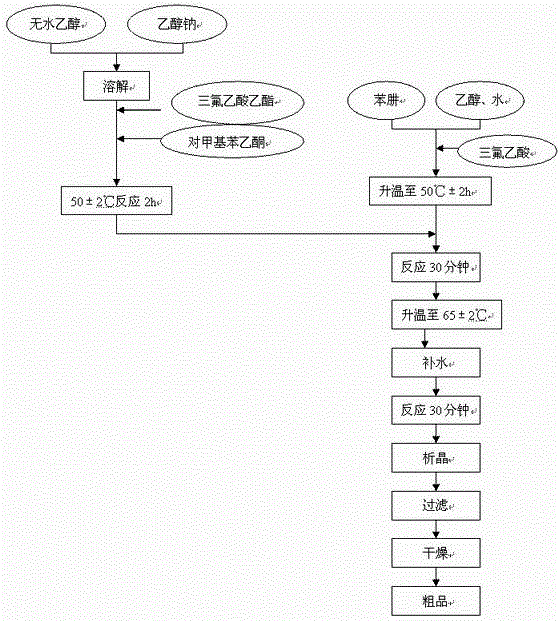
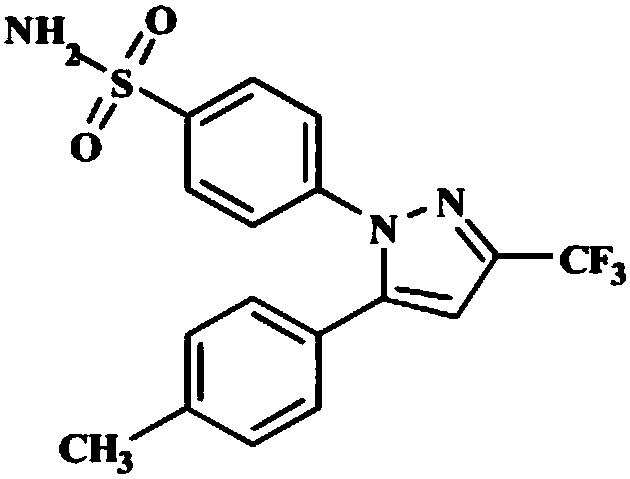
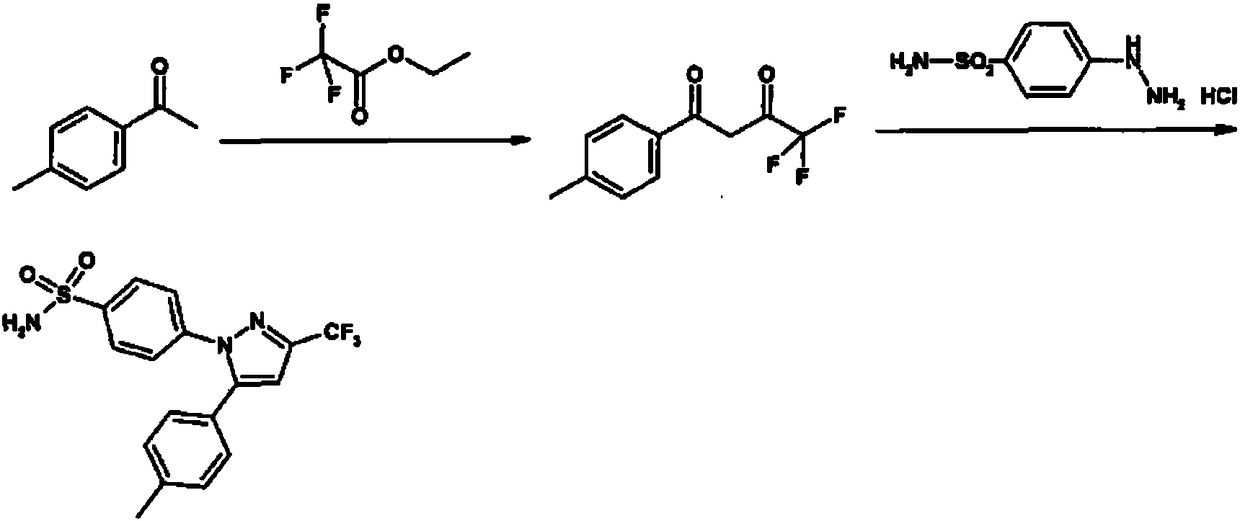
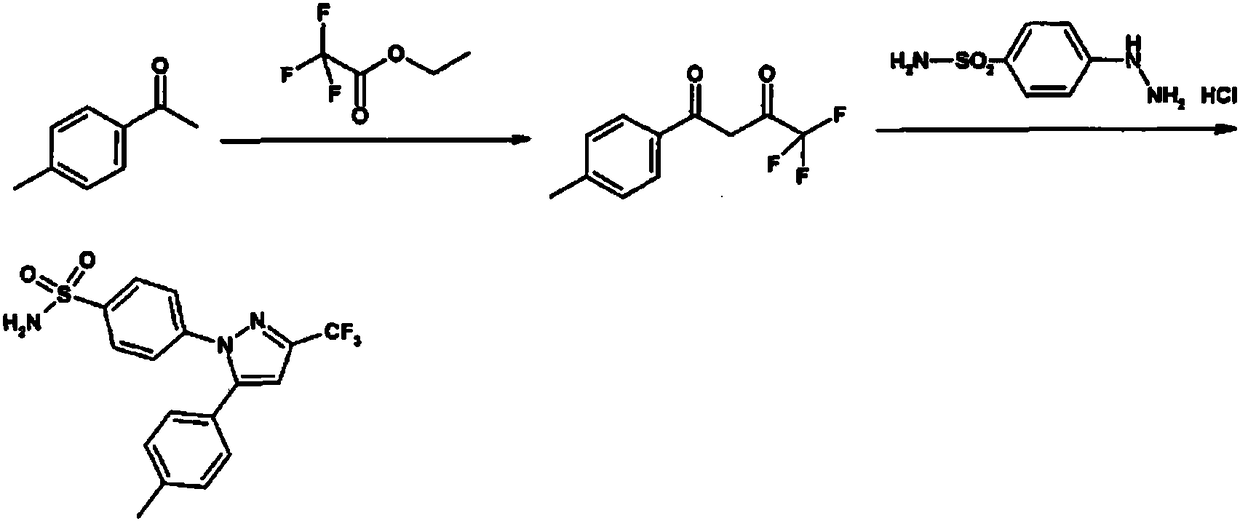
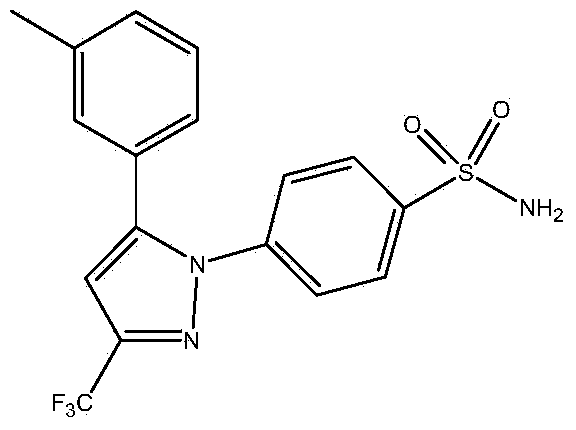
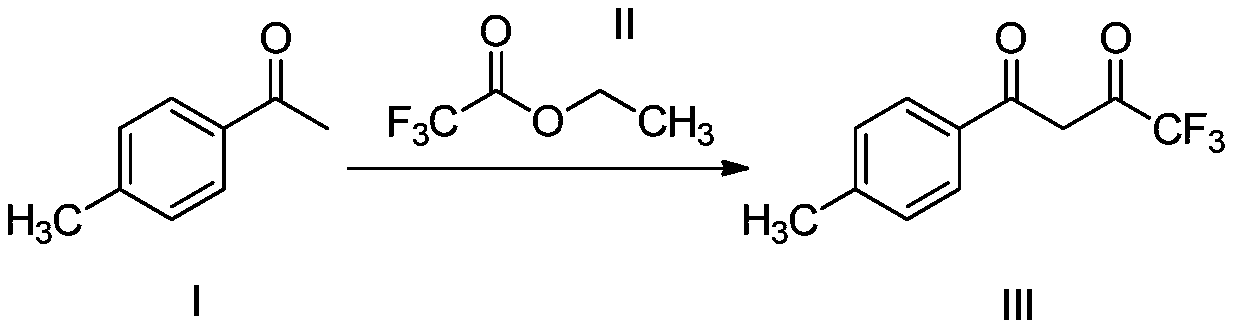
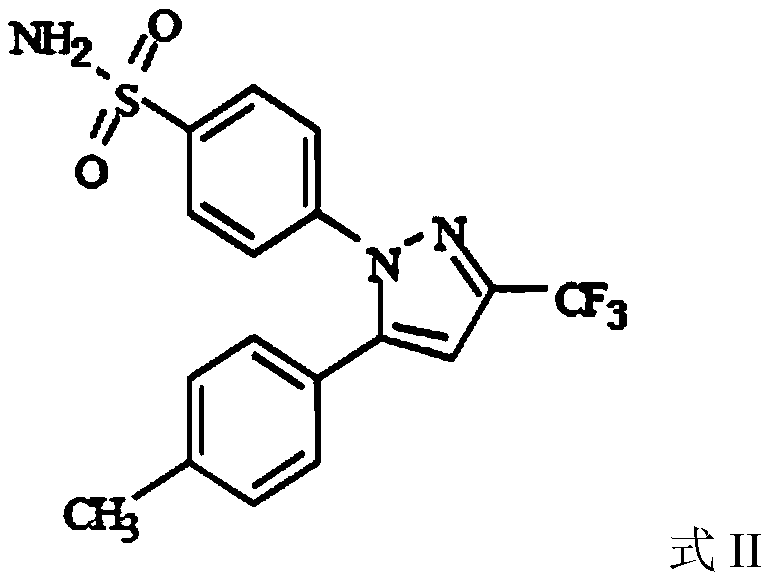
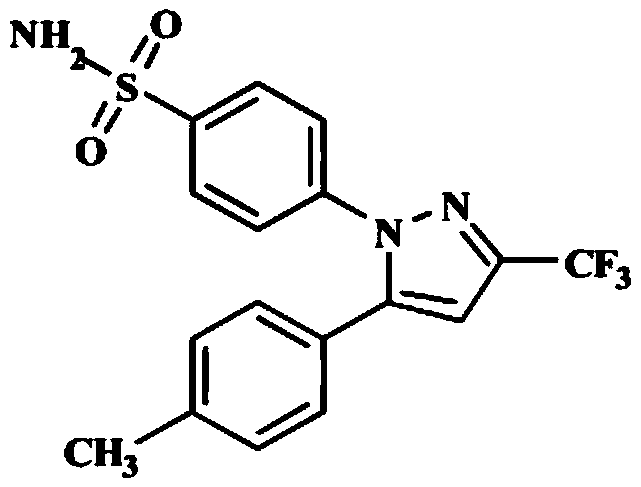
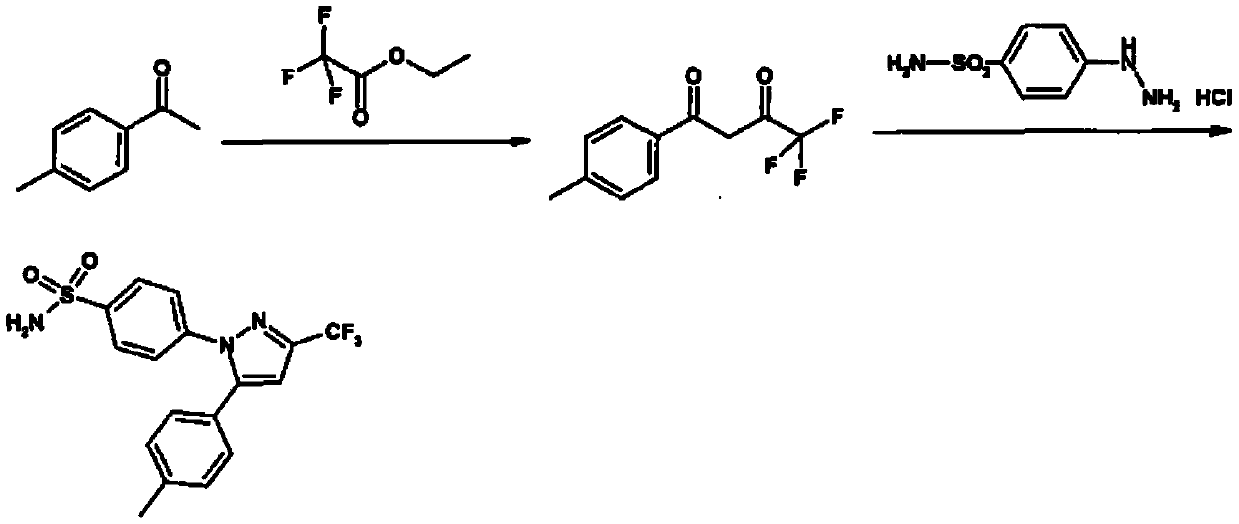
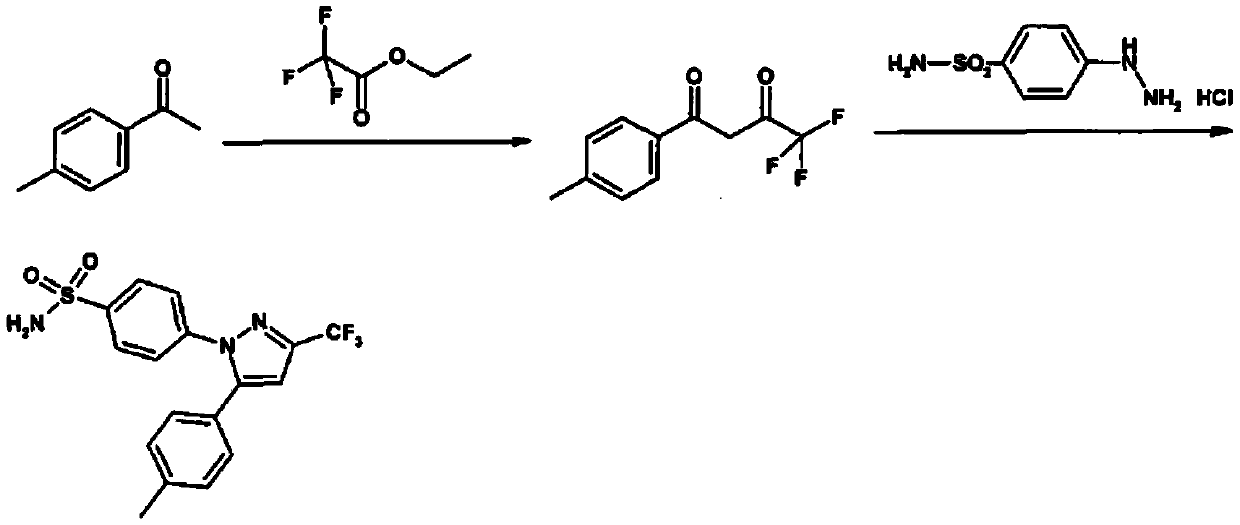
The invention relates to a synthesis method of celecoxib, which comprises the following specific steps of: 1, carrying out claisen condensation on p-methylacetophenone and trifluoroacetic acid ethyl esters in an aprotic organic solvent under the catalysis of alkali to obtain 1-(4-methylphenyl)-4,4,4-trifluoro-1,3-butanedione; and 2, reacting the obtained 1-(4-methylphenyl)-4,4,4-trifluoro-1,3-butanedione with sulfonamide-phenylhydrazine hydrochloride to obtain celecoxib, wherein in the step 1, the alkali for catalysis is selected from one or more of sodium hydride, potassium hydride, lithium hydride and calcium hydride. The synthesis method of celecoxib provided by the invention is easy to operate, high in yield, high in product purity and easy for industrial production.
Owner:JIANGXI SYNERGY PHARMA
Celecoxib preparation method
InactiveCN105130901AFine granularitySuitable for productionOrganic chemistryClaisen condensationAcetophenone
The present invention relates to a celecoxib preparation method, wherein 4'-methylacetophenone and ethyl trifluoroacetate are adopted as raw materials, Claisen condensation is performed to obtain a beta-diketone intermediate, the beta-diketone intermediate and p-hydrazinobenzenesulfonamide hydrochloride are subjected to condensation cyclization in ethanol to obtain celecoxib, and refining and crystallization are performed to obtain the celecoxib crystal. According to the present invention, with the preparation method, the celecoxib can be obtained in the high yield manner, the purity of the obtained product is high, the single impurity can be controlled to be less than or equal to 0.5%, and the obtained celecoxib crystal has characteristics of fine particle size and uniform distribution, and is suitable for bulk drug production.
Owner:SUZHOU ERYE PHARMA CO LTD
Preparation method and application of carbonyl hydrogenation catalyst
ActiveCN110227487AImprove the defect of a single surface active siteMany surface active sitesOrganic compound preparationHydroxy compound preparationActivated carbonHydrogenation reaction
The invention discloses a preparation method of a carbonyl hydrogenation catalyst. The method comprises the steps of: cooking activated carbon with hydrochloric acid, and performing filtering and washing; cooking the acid treated activated carbon with an alkaline solution, and performing filtering and washing; cooking the alkali treated activated carbon with an oxidizing agent solution, and performing filtering and washing; adding the activated carbon subjected to oxidation treatment into a modifier solution, and performing stirring to obtain a modified activated carbon slurry; mixing a palladium chloride solution with a copper salt solution, adding the mixed solution of palladium chloride and copper salt into the activated carbon slurry, and performing stirring to obtain a loaded palladium-copper suspension precursor; and reducing the loaded palladium-copper suspension precursor, and conducting filtering, washing and centrifugal drying to obtain the carbonyl hydrogenation catalyst. Inaddition, the invention also provides application of the carbonyl hydrogenation catalyst prepared by the above method in the hydrogenation reaction of p-methylacetophenone. The preparation method provided by the invention is simple, and the carbonyl hydrogenation catalyst prepared by the method has high catalytic activity in the hydrogenation reaction of p-methylacetophenone.
Owner:XIAN CATALYST NEW MATERIALS CO LTD
Flavor deterioration inhibitor and inhibitor for the generation of citral deterioration smell
InactiveUS20060062813A1Improve securityInhibit flavorCosmetic preparationsToilet preparationsPlantago asiaticaFood flavor
A flavor deterioration inhibitor which comprises an extract obtained by extracting Angelica keiskei, avocado, Cassia tora, Plantago asiatica L, hawthorn, fermented tea leaves or semi-fermented tea leaves with water, an organic polar solvent or a mixture thereof; and a deterioration smell inhibitor for citral or a citral-containing product. By adding the above flavor deterioration inhibitor to foods, drinks or oral care products, it is possible to inhibit the deterioration of a flavor which is easily affected by light, heat, oxygen and soon. In particular, a remarkable inhibitory effect can be achieved on deterioration due to light. By blending the above deterioration smell inhibitor with ctiral or a citral-containing product, the generation of the deterioration smell (caused by p-cresol and p-methylacetophenone) due to the passage of time or heating can be effectively inhibited.
Owner:OGAWA & CO LTD
Synthetic method for 2-cyano-4-chloro-5-(4-methylphenyl)imidazole
The invention relates to a synthetic method for 2-cyano-4-chloro-5-(4-methylphenyl)imidazole. The synthetic method comprises the following steps: (1) by taking p-methylacetophenone as a raw material, carrying out a halogenating reaction under a light condition to obtain a compound I; (2) dissolving the obtained compound I in a solvent, stirring the mixture to react at a certain temperature, and performing cooling and suction-filtering to obtain a compound II; (3) dissolving the obtained compound II in a solvent and carrying out a reaction with glyoxal and hydroxylamine sulphate at a certain temperature to obtain a compound III; and (4) dissolving the obtained compound III in a solvent, carrying out a reaction with sulfoxide chloride in an ice bath in a manner of raising the temperature to room temperature and keeping the temperature for the reaction, dropwise adding sulfur chloride, and after reaction, washing the product to obtain a compound IV which is 2-cyano-4-chloro-5-(4-methylphenyl)imidazole. The synthetic method is good in atom economy, simple and convenient in process operation and high in product yield and industrial application value.
Owner:RUDONG ZHONGYI CHEM
Method for producing p-methylacetophenone with fixed-bed reactor
InactiveCN103130633AEasy to operateSimple structureCarbonyl compound preparation by condensationFixed bedToluene
The invention discloses a method for producing p-methylacetophenone with a fixed-bed reactor, which comprises the following steps: (1) reaction section: under the conditions of 60-300 DEG C and 0.05-30 MPa, evenly mixing toluene and an acylation reagent, sending the mixture into a fixed-bed reactor by a raw material feeding pump to carry out acylation reaction, thereby obtaining a p-methylacetophenone crude product, wherein the fixed-bed reactor is filled with a catalyst bed layer; and (2) refinement section: purifying the p-methylacetophenone crude product by a rectification technique to obtain the high-purity p-methylacetophenone product. Compared with the traditional technical process, the technical process disclosed by the invention is simple to operate, and has the advantages of low investment cost, high product purity and the like; and meamwhile, the reactor has a simple structure, is stable for operation and convenient for control, and can easily implement large-scale and continuous production.
Owner:TIANJIN UNIV
Method for preparing celecoxib by using one-pot method
ActiveCN108558759AReduced responseGood effect of removing impuritiesOrganic chemistryEthylenediamineFiltration
The invention discloses a method for preparing celecoxib by using a one-pot method. The method comprises the following steps: in the presence of ethidene diamine, mixing p-methylacetophenone and ethyltrifluoroacetate, and enabling the components to react completely at 40-80 DEG C without other solvent so as to obtain a reaction liquid of an intermediate DO; putting the reaction liquid into an alcohol solvent, further adding bihydrazino-benzsulfamide hydrochloride, further adding an organic acid to adjust the pH value to 3-6, controlling the temperature of a material liquid to 50-80 DEG C, andenabling the components to react completely; after the reaction is completed, adding water, cooling to 10-30 DEG C to separate out a crystal, and carrying out suction filtration so as to obtain a crude product of celecoxib; dissolving the crude product with methanol, dropping the material liquid into water, controlling the temperature of the material liquid to 40-50 DEG C in the dropping process,cooling to 10-30 DEG C to separate out a crystal after dropping is completed, and carrying out suction filtration, thereby obtaining a finished product of celecoxib. The total yield of the product prepared by using the method is greater than 85%, and HPLC (High Performance Liquid Chromatography) tests show that the purity of the product is greater than or equal to 99.90%.
Owner:山东安信制药有限公司
Preparation method of celecoxib
InactiveCN104177294AMild reaction conditionsEasy to operateOrganic chemistryDissolutionEthyl acetate
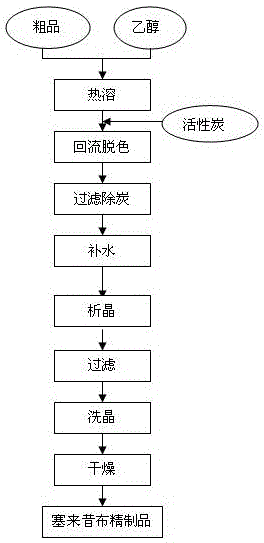
The invention discloses a preparation method of celecoxib. The method comprises the steps of adding ethyl trifluoroacetate and sodium alcoholate in an organic solvent, adding p-methylacetophenone to carry out reaction at 40-50 DEG C for 1-2h and adding petroleum ether to carry out devitrification after the reaction so as to obtain a midbody 1; mixing the midbody 1 with ethyl acetate and isopropyl alcohol, adding hydrochloric acid and sulfonamidophenylhydrazine hydrochloride, controlling the temperature within 50-80 DEG C to carry out reaction for 1-2h and adding water to carry out devitrification after the reaction so as to obtain crude celecoxib; and adding the crude celecoxib in ethyl alcohol to carry out heating dissolution, adding activated carbon to carry out decoloration, cooling to 10-30 DEG C and adding water to carry out devitrification so as to obtain the finished product celecoxib. The preparation method has the advantages of mild reaction conditions, high yield, good purity, simplicity in operation and environment friendliness, has a more secure and environment-friendly synthesis process and wide market prospect and economic benefit.
Owner:QILU TIANHE PHARMA
Flavor deterioration inhibitor and inhibitor for the generation of citral deterioration smell
A flavor deterioration inhibitor which comprises an extract obtained by extracting Angelica keiskei, avocado, Cassia tora, Plantago asiatica L, hawthorn, fermented tea leaves or semi-fermented tea leaves with water, an organic polar solvent or a mixture thereof; and a deterioration smell inhibitor for citral or a citral-containing product. By adding the above flavor deterioration inhibitor to foods, drinks or oral care products, it is possible to inhibit the deterioration of a flavor which is easily affected by light, heat, oxygen and so on. In particular, a remarkable inhibitory effect can be achieved on deterioration due to light. By blending the above deterioration smell inhibitor with ctiral or a citral-containing product, the generation of the deterioration smell (caused by p-cresol and p-methylacetophenone) due to the passage of time or heating can be effectively inhibited.
Owner:OGAWA & CO LTD
Synthetic method of COX-2 enzyme inhibitor celecoxib intermediate
ActiveCN103951549AReduce consumptionMild reaction conditionsOrganic compound preparationCarbonyl compound preparation by condensationOrganic solventTrifluoroacetic acid
The invention belongs to the technical field of clean synthesis and particularly relates to a synthetic method of a COX-2 enzyme inhibitor celecoxib intermediate. The method comprises the following steps: reacting initial raw materials (ethyl trifluoroacetate (II) and p-methylacetophenone (I)) in the presence of carbonate serving as alkali in an organic solvent and obtaining the celecoxib intermediate 4,4,4-trifluoro-(4-methylphenyl)-1,3-butanedione (III) with the yield of 83%-99%. In the whole process, the raw material consumption is low, alkali and a solvent can be recycled, and the generation of three wastes is low; and the method has good industrial prospect.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
Chilo suppressalis male insect trapping agent and preparation method thereof
InactiveCN112352781AExtended release timeImprove trapping effectBiocidePest attractantsSexual PheromonesCaryophyllene
The invention discloses a chilo suppressalis trapping agent and a preparation method thereof. The chilo suppressalis trapping agent is prepared from, by weight, 10-15 parts of synergist, 50-70 parts of sex pheromone and 600-900 parts of a solvent; the synergist is a combination of beta- caryophyllene, 4'-Methylacetophenone and n-nonane; the sex pheromone is a combination of cis-11-hexedecanal, cis-13-octadecenal and cis-9-hexedecanal. Through combination of the synergist and the sex pheromone, the trapping amount of chilo suppressalis male insects can be greatly increased, and the release timeof the synergist can be prolonged. The synergist is beta-caryophyllene, 4'-Methylacetophenone and n-nonane, but for a single-component synergist, the combined synergist can enhance the trapping effect on chilo suppressalis. The optimized chilo suppressalis sex pheromone composite synergist can trap a large number of chilo suppressalis adults in the field. The technology is simple and convenient to operate, and can be applied to chilo suppressalis prediction and green prevention and control.
Owner:贵州省植物保护研究所 +1
Perfume Compositions
InactiveUS20090004303A1Relieve pressureEasy to relaxBiocideCosmetic preparationsLife qualityAdditive ingredient
Perfume ingredients and essential oils in synergistic combinations improve well-being by down-regulating arousal. This could lead to enhanced quality of life and reduced stress. The perfume compositions of the invention comprise one or more materials from each of the following groups: Group A: the essential oils lavender, lavandin, bergamot, chamomile, clary sage; Group B: 2-phenoxyethanol, 1-(2,3,8,8-tetramethyl-1,2,3,4,5,6,7,8-octahydronaphthalen-2-yl)ethanone, benzophenone, cyclopentadecanolide, alpha-ionone, beta-ionone, para-methylacetophenone, [4-isopropylcyclohexyl]methanol. The ratio of the weight percentage based on the composition of group A materials to Group B materials lies within the range 1:9 to 9:1.
Owner:QUEST INTERNATIONAL
Tea fragrance type cigarette essence and application thereof
InactiveCN109055009ASmoke is soft and delicateReduce stimulationTobacco preparationEssential-oils/perfumesPhenethyl alcoholFood science
The invention discloses a tea fragrance type cigarette essence which is prepared by mixing the following components in parts by weight: 15-20 parts of 75% by mass of alcohol, 12-20 parts of Anji whitetea extract, 10-15 parts of pubescent holly root extract, 5-11 parts of masson pine needle extract, 0.1-0.3 part of beta-phenethyl alcohol, 1-3 parts of terpene-containing sweet orange oil, 0.02-0.05part of p-methylacetophenone, 0.05-0.08 part of allyl hexanoate, 0.06-0.10 part of isoamyl phenylacetate and the balance of propylene glycol till 100 parts. The tea fragrance type cigarette essence accounting for 0.01-0.05% of the mass of tobacco shreds is added into tobacco shreds of cigarette. The tea fragrance type cigarette essence disclosed by the invention has fresh, thick and long-lastingtea fragrance, is rich and full in tobacco fragrance, fine and gentle in cigarette smell and capable of effectively reducing irritant and impure smell, has sweet aftertaste and is capable of promotingsecretion of fluids.
Owner:CHINA TOBACCO ZHEJIANG IND
A kind of synthetic method of 4-chloro-2-cyano-n,n-dimethyl-5-(4'-methylphenyl)-1h-imidazole-1-sulfonamide
ActiveCN103936678BShort reaction cycleSimple post-processingOrganic chemistryN dimethylformamideSynthesis methods
The invention discloses a synthesis method of 4-chloro-2-cyano-N,N-dimethyl-5-(4-methylphenyl)-1H-imidazole-1-sulfonamide (cyazofamid). According to the synthesis method, methylacetophenone is taken as a raw material, selenium dioxide is used for oxidation to prepare an intermediate, namely 2-carbonyl-2-p-benzyl aldehyde, then N,N-dimethylformamide and the like are taken as solvents, thionyl chloride is taken as a chlorinating agent and reducing agent for preparing the intermediate, and then intermediate further reacts with N,N-dimethyl sulfonamide chloride to synthesize the cyazofamid. Compared with the prior art, the synthesis method has the advantages that the reaction period is shortened, the post-treatment process is simplified and the synthesis method is suitable for mass industrial production.
Owner:XIAN MODERN CHEM RES INST
Synthetic method of celecoxib
InactiveCN103923011AImprove solubilityImprove recycling effectOrganic chemistryPhenylhydrazine hydrochlorideFiltration
The invention discloses a synthetic method of celecoxib. The synthetic method is characterized by comprising the following steps: firstly, adding sodium methylate as an aldol condensation catalyst and methylbenzene as a reaction solvent into a reaction container, adding p-methylacetophenone and trifluoroacetic acid ethyl ester, fully reacting, adding diluted hydrochloric acid, and separating out a water layer to obtain a methylbenzene solution of an intermediate-diketone; secondly, adding water, a phase transfer catalyst and 4-aminosulfophenyl hydrazine hydrochloride into the methylbenzene solution of the intermediate-diketone, and performing dehydration cyclization reaction to obtain a celecoxib reaction solution; thirdly, replenishing methylbenzene, standing, separating out the water layer and remaining an organic layer; cooling the organic layer, preserving heat and crystallizing to obtain a crystal product, and performing suction filtration, methylbenzene washing and water washing in sequence to obtain a celecoxib crude product; drying the crude product in vacuum, decolorizing and re-crystallizing to obtain a celecoxib raw material. According to the synthetic method, the celecoxib yield is increased, the reaction time is shortened, the celecoxib purity is high, the three-waste discharge in the whole production process is reduced, and the production cost is low.
Owner:SUZHOU TIANMA SPECIALTY CHEM
Degradation inhibitor for flavor or aroma
ActiveUS20100189822A1Inhibit deterioration of flavor and fragranceKeep the flavorCosmetic preparationsBiocideAdditive ingredientFood flavor
The invention provides a deterioration inhibitor with an even stronger effect than the prior art against deterioration of flavors and fragrances in foods and beverages or cosmetics, which is attributed to heat, light and the like, and especially against production of deterioration odors derived from citral.It is a food flavor deterioration inhibiting material comprising as an effective component a product obtained by treatment of a tea extract component with an oxidizing enzyme. Addition of a deterioration inhibiting material according to the invention to a food or beverage or a cosmetic can inhibit deterioration of flavor and fragrance, with use in smaller amounts than conventional deterioration inhibitors. A particularly notable effect is exhibited against production of p-cresol and p-methylacetophenone which are citral-derived deterioration odor components, and therefore it is suitable for foods and beverages or cosmetics with citral-containing citrus-like flavors and fragrances.
Owner:OGAWA & CO LTD
Synthesis method of high-purity histamine dihydrochloride
ActiveCN112266360AImprove the coordination effectLower the decarboxylation temperatureOrganic chemistryMethylanilineDimethylaniline N-oxide
The invention discloses a synthesis method of high-purity histamine dihydrochloride, and belongs to the technical field of organic synthesis. The method comprises the steps: in a solvent A, carrying out a decarboxylation reactionon L-histidine at the temperature of 110-150 DEG C under the effect of a composite decarboxylation catalyst, and carrying out filtering after the reaction is completed, wherein the composite decarboxylation catalyst is composed of a main catalyst and an auxiliary catalyst, the main catalyst is selected from sodium hydroxide, potassium hydroxide, sodium carbonate, potassium carbonate, pyridine, 4-methylpyridine, aniline, 4-methylaniline, ,N,N-dimethylaniline and N, N-dimethylformamide, and the auxiliary catalyst is one selected from benzophenone, acetophenone, benzophenone and p-methyl acetophenone; carrying out reduced pressure distillation on a filtrate, adding water into residues, and adjusting the pH value to 5-6 by using hydrochloric acid to obtain an aqueous solution; extracting the aqueous solution at least once by using an extracting agent to remove impurities; and evaporating to remove water in the aqueous solution, pulping with a solvent B, filtering and drying to obtain the product.
Owner:WUHAN JASON BIOTECH CO LTD
Method for synthesizing loxoprofen sodium
ActiveCN101412670BRaw materials are easy to getUnique craftOrganic compound preparationCarboxylic compound preparationSolventHydrolysis
The invention discloses a synthetic method for loxoprofen sodium, which is prepared by taking methyl acetophenone as an initial raw material through reduction, acylation or halogen substituent, cyanation, hydrolysis, bromination, condensation, decarboxylation and salifying. The method has the advantages of easily obtained raw material, unique technology, simple and stable operation, and high productive rate in each step of reaction; and all solvents used in the synthesis process can be recycled, so the production cost is reduced greatly. Tests show that the obtained product has reliable quality and stable performance, and can be further used for making preparation of non-steroidal anti-inflammatory drugs such as the loxoprofen sodium.
Owner:ZHEJIANG APELOA JIAYUAN PHARMA +1
Essence having green grass fragrance
Essence having green grass fragrance is disclosed. The essence includes, by mass, 1-3 parts of p-methylacetophenone, 18-21 parts of butyl salicylate, 4-6 parts of isobutyl phenylacetate, 4-6 parts of bergamot oil, 19-21 parts of geranium oil, 5-6 parts of anisic aldehyde, 30-32 parts of lavandula hybrida oil, 3-5 parts of cananga odorata oil, 2-4 parts of cablin patchouli herb oil, 3-5 parts of oakmoss extract and 3-5 parts of coumarin. The essence has natural and fresh green grass fragrance, a strong natural sense, long duration time and obvious characteristic fragrance, can be widely applied in daily chemical products, and can meet ever-growing material and cultural needs of the people.
Owner:安徽香杰香精科技有限公司
Method for preparing Celecoxib dione intermediate
InactiveCN110407683AOrganic compound preparationCarbonyl compound separation/purificationReaction temperatureSolvent
The invention provides a method for preparing a Celecoxib dione intermediate. The method comprises the steps: subjecting p-methylacetophenone and ethyl trifluoroacetate to a reaction in the presence of sodium methylate, wherein toluene serves as a solvent, and a reaction temperature is 20 DEG C to 40 DEG C; carrying out quenching with a hydrochloric solution after the reaction is complete, then, carrying out washing with purified water, and carrying out standing to separate out an aqueous phase; and subjecting an organic phase to cooling crystallization, thereby obtaining a yellowish solid product. According to the method provided by the invention, the safety of industrial production of the Celecoxib dione intermediate can be improved, the process is very convenient, and meanwhile, the purity and yield of the product are not lowered.
Owner:HUNAN FANGSHENG PHARMACEUTICAL CO LTD
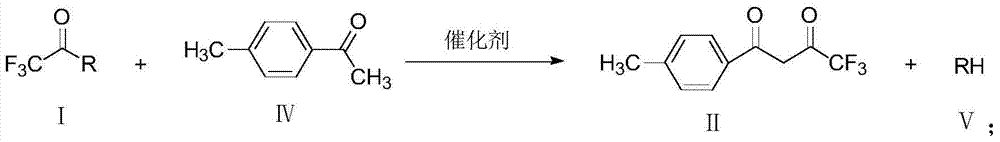
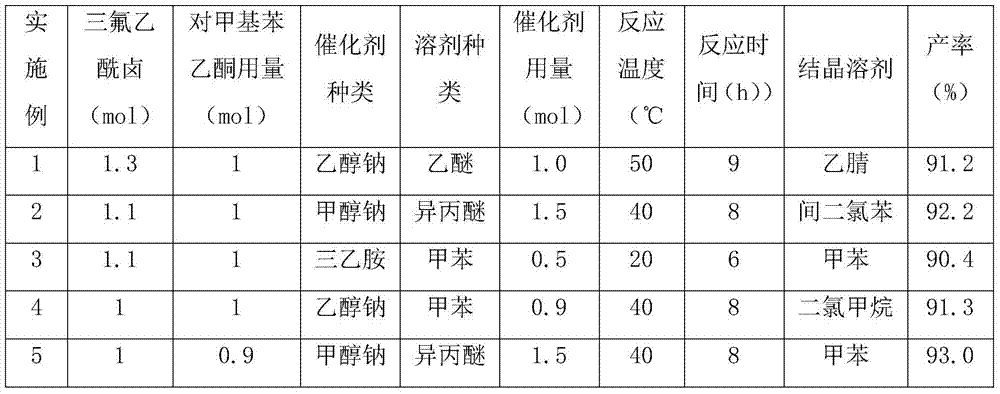
A method for preparing 1-p-methylphenyl-4,4,4-trifluoro-1,3-butanedione
ActiveCN106892806BReduce pollutionLow costOrganic compound preparationCarbonyl compound preparationPtru catalystOrganic solvent
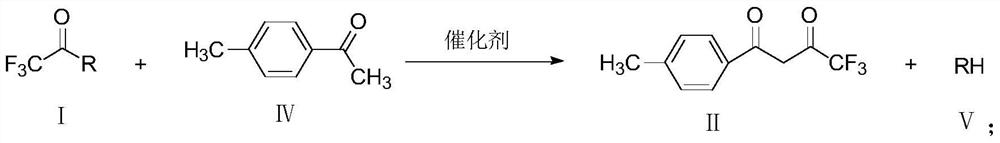
The invention provides a method for preparing 1-p-methylphenyl-4,4,4-trifluoro-1,3-butanedione. The method comprises the step of reacting by virtue of trifluoroacetyl halide and p-methylacetophenone in an organic solvent under the action of a catalyst, so as to obtain 1-p-methylphenyl-4,4,4-trifluoro-1,3-butanedione and / or an enol formisomer of 1-p-methylphenyl-4,4,4-trifluoro-1,3-butanedione. The method provided by the invention has the advantages of low raw material cost, mild reaction conditions, low environmental pollution and suitability in industrial production.
Owner:ZHEJIANG LANTIAN ENVIRONMENTAL PROTECTION HI TECH +1
Preparation method of 4-acetylbenzoic acid
InactiveCN102701965AReduce dosageReduce manufacturing costOrganic compound preparationCarboxylic compound preparationCooking & bakingAcetic acid
The invention discloses a preparation method of 4-acetylbenzoic acid. The preparation method comprises the following steps of: (1) oxidizing: adding 4-methyl acetophenone, water and anhydrous zinc chloride to a reaction pot, stirring uniformly, slowly heating to 35-45 DEG C, averagely dividing potassium permanganate into five parts, adding one part every 15-20min, controlling the reaction temperature at 48-55 DEG C during adding, after adding is finished, controlling the reaction temperature at 40-45 DEG C, carrying out heat insulation for 1.5h, then cooling to 17-22 DEG C, centrifuging, and baking to obtain 4-acetylbenzoic acid crude product; and (2) mixing prepared crude product with anhydrous acetic acid, heating and refluxing for 0.5-1.5h, carrying out hot filtering, centrifuging and baking to obtain 4-acetylbenzoic acid. The method has the advantages that the production cost is low, batched oxidation is adopted, the solvent consumption is reduced, the discharge of three wastes is reduced, the production process is environment-friendly and the yield is high.
Owner:扬州市天平化工厂有限公司
Method for preparing 1-p-methylphenyl-4,4,4-trifluoro-1,3-butanedione
ActiveCN106892806AReduce pollutionLow costOrganic compound preparationCarbonyl compound preparationOrganic solventP-methylacetophenone
The invention provides a method for preparing 1-p-methylphenyl-4,4,4-trifluoro-1,3-butanedione. The method comprises the step of reacting by virtue of trifluoroacetyl halide and p-methylacetophenone in an organic solvent under the action of a catalyst, so as to obtain 1-p-methylphenyl-4,4,4-trifluoro-1,3-butanedione and / or an enol formisomer of 1-p-methylphenyl-4,4,4-trifluoro-1,3-butanedione. The method provided by the invention has the advantages of low raw material cost, mild reaction conditions, low environmental pollution and suitability in industrial production.
Owner:ZHEJIANG LANTIAN ENVIRONMENTAL PROTECTION HI TECH +1
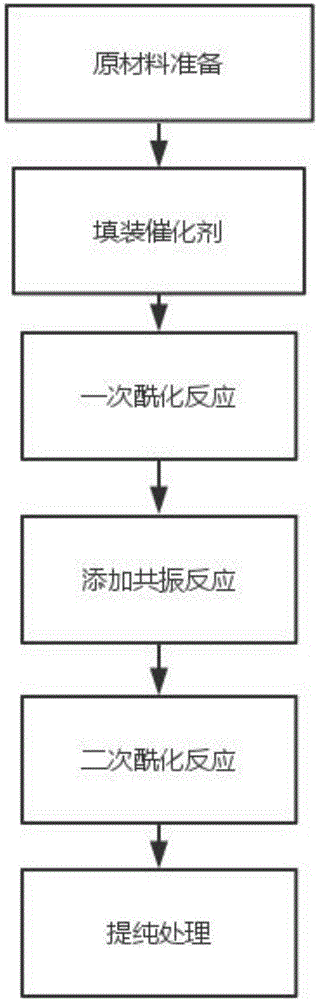
Method for preparing p-methylacetophenone
InactiveCN106380386AHigh puritySimple structureCarbonyl compound preparation by condensationCarbonyl compound separation/purificationMaterials preparationResonance
The invention discloses a method for preparing p-methylacetophenone, which relates to the field of pesticide production. The method comprises six processes of raw materials preparation, filling of a catalyst, primary acylation reaction, addition of a resonance reaction, secondary acylation and purification processing. The method takes toluene and an acylating agent as the main raw materials, the raw materials are subjected to an acylation reaction to obtain p-methylacetophenone, one more time of the an acylation reaction flow is added based on traditional technological foundation, the method comprises the primary acylation reaction and secondary acylation reaction, and the p-methylacetophenone with higher purity can be obtained through the step-by-step acylation reaction. In the preparation method, a reactor has the advantages of simple structure, stable operation, convenient control, and easy realization of large scale and continuous production, and is worth of popularization.
Owner:枣阳市先飞高科农药有限公司
A kind of synthetic method of cox-2 enzyme inhibitor celecoxib intermediate
ActiveCN103951549BReduce consumptionMild reaction conditionsOrganic compound preparationCarbonyl compound preparation by condensationOrganic solventSynthesis methods
The invention belongs to the technical field of clean synthesis and particularly relates to a synthetic method of a COX-2 enzyme inhibitor celecoxib intermediate. The method comprises the following steps: reacting initial raw materials (ethyl trifluoroacetate (II) and p-methylacetophenone (I)) in the presence of carbonate serving as alkali in an organic solvent and obtaining the celecoxib intermediate 4,4,4-trifluoro-(4-methylphenyl)-1,3-butanedione (III) with the yield of 83%-99%. In the whole process, the raw material consumption is low, alkali and a solvent can be recycled, and the generation of three wastes is low; and the method has good industrial prospect.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
Degradation inhibitor for flavor or aroma
ActiveUS9028886B2Inhibit deterioration of flavor and fragranceKeep the flavorBiocideCosmetic preparationsFlavorAroma aroma
The invention provides a deterioration inhibitor containing an amount of a product obtained by treatment of a tea extract with an oxidizing enzyme and then inactivating the enzyme. Addition of a deterioration inhibiting material according to the invention to a food or beverage or a cosmetic can inhibit deterioration of flavor and fragrance, with use in smaller amounts than conventional deterioration inhibitors. A particularly notable effect is exhibited against production of p-cresol and p-methylacetophenone which are citral-derived deterioration odor components, and therefore it is suitable for foods and beverages or cosmetics with citral-containing citrus-like flavors and fragrances.
Owner:OGAWA & CO LTD
Flavor deterioration inhibitor and inhibitor for the generation of citral deterioration smell
A flavor deterioration inhibitor which comprises an extract obtained by extracting Angelica keiskei, avocado, Cassia tora, Plantago asiatica L, hawthorn, fermented tea leaves or semi-fermented tea leaves with water, an organic polar solvent or a mixture thereof; and a deterioration smell inhibitor for citral or a citral-containing product. By adding the above flavor deterioration inhibitor to foods, drinks or oral care products, it is possible to inhibit the deterioration of a flavor which is easily affected by light, heat, oxygen and so on. In particular, a remarkable inhibitory effect can be achieved on deterioration due to light. By blending the above deterioration smell inhibitor with ctiral or a citral-containing product, the generation of the deterioration smell (caused by p-cresol and p-methylacetophenone) due to the passage of time or heating can be effectively inhibited.
Owner:OGAWA & CO LTD
One-pot method for preparing celecoxib
ActiveCN108558759BReduced responseGood effect of removing impuritiesOrganic chemistryPhenylsulfonamideAcetophenone
The invention discloses a method for preparing celecoxib by using a one-pot method. The method comprises the following steps: in the presence of ethidene diamine, mixing p-methylacetophenone and ethyltrifluoroacetate, and enabling the components to react completely at 40-80 DEG C without other solvent so as to obtain a reaction liquid of an intermediate DO; putting the reaction liquid into an alcohol solvent, further adding bihydrazino-benzsulfamide hydrochloride, further adding an organic acid to adjust the pH value to 3-6, controlling the temperature of a material liquid to 50-80 DEG C, andenabling the components to react completely; after the reaction is completed, adding water, cooling to 10-30 DEG C to separate out a crystal, and carrying out suction filtration so as to obtain a crude product of celecoxib; dissolving the crude product with methanol, dropping the material liquid into water, controlling the temperature of the material liquid to 40-50 DEG C in the dropping process,cooling to 10-30 DEG C to separate out a crystal after dropping is completed, and carrying out suction filtration, thereby obtaining a finished product of celecoxib. The total yield of the product prepared by using the method is greater than 85%, and HPLC (High Performance Liquid Chromatography) tests show that the purity of the product is greater than or equal to 99.90%.
Owner:山东安信制药有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com