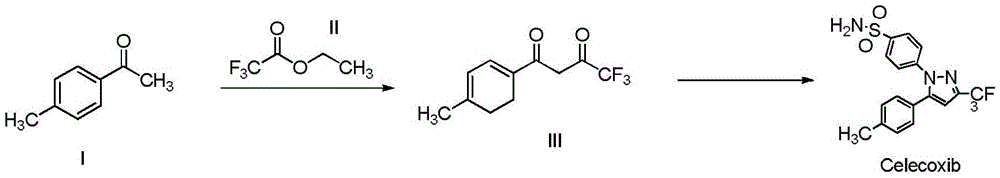
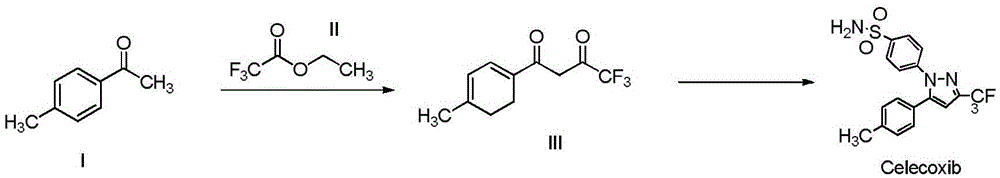
A kind of synthetic method of cox-2 enzyme inhibitor celecoxib intermediate
A technology of COX-2 and celecoxib, applied in chemical instruments and methods, condensation preparation of carbonyl compounds, preparation of carbon-based compounds, etc., can solve environmental problems of industrial wastewater, strong alkalinity of sodium methoxide, easy hydrolysis and other problems, Achieve broad market prospects and economic benefits, simple operation, and low pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Add 200mL of ethanol, 200mmol (26.8mL) of p-methylacetophenone (I), 600mmol (72mL) of ethyl trifluoroacetate (II), 360mmol of anhydrous potassium carbonate in a 500mL single-port bottle, and react at -20°C 28h. Potassium carbonate and potassium bicarbonate were recovered by suction filtration. The mother liquor is rectified to recover the solvent and unreacted raw materials. Add an equal volume of water to the rectification raffinate, adjust the pH value to 6 with 10% hydrochloric acid, extract 4 times with petroleum ether, 70 mL each time, combine the organic phases, concentrate, and freeze-dry to obtain 44.3 g of light yellow solid product (III) , the yield was 96.3%.
Embodiment 2
[0028] Add 200mL of acetonitrile-isopropanol (1:1), 200mmol (26.8mL) of p-methylacetophenone (I), 600mmol (72mL) of ethyl trifluoroacetate (II), and 360mmol of Potassium carbonate with a diameter of 600nm was reacted at 40°C for 24h. Potassium carbonate and potassium bicarbonate are recovered by suction filtration, and can be reused after high temperature treatment. The mother liquor is rectified to recover the solvent and unreacted raw materials for the next reaction. Add an equal volume of water to the rectification raffinate, adjust the pH value to 6 with 10% hydrochloric acid, extract 4 times with ethyl acetate, 70 mL each time, combine the organic phases, concentrate and lyophilize to obtain a light yellow solid product (III) 45.6 g, the yield is 99.08%.
Embodiment 3
[0030] Add 200mL of ethylene glycol dimethyl ether, 200mmol (26.8mL) of p-methylacetophenone (I), 600mmol (72mL) of ethyl trifluoroacetate (II), and 360mmol of carbonic acid with a particle size of 200nm in a 500mL single-port bottle. Potassium, reflux reaction for 16h. Potassium carbonate and potassium bicarbonate are recovered by suction filtration, and can be reused after high temperature treatment. The mother liquor is rectified to recover the solvent and unreacted raw materials for the next reaction. Add an equal volume of water to the rectification raffinate, adjust the pH value to 6 with 10% hydrochloric acid, extract 4 times with diethyl ether, 70 mL each time, combine the organic phases, concentrate, and lyophilize to obtain 44.8 g of a pale yellow solid product (III). The yield was 97.4%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com