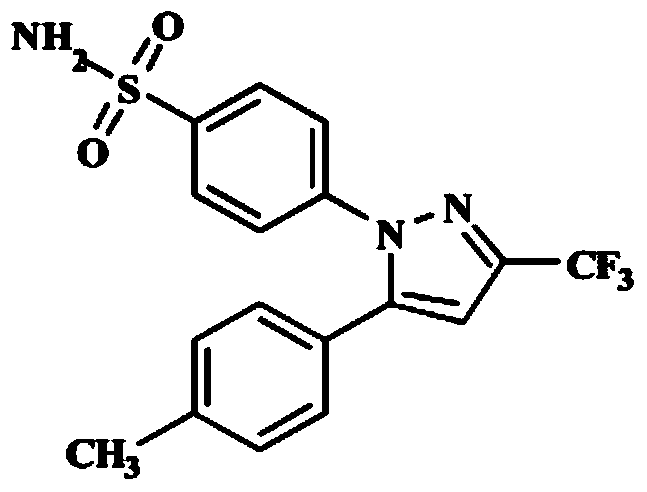
Preparation method of celecoxib
A technology of celecoxib and sodium alkoxide, which is applied in the field of medicine, can solve problems such as unqualified solvent residues in products, expensive mixed solutions, and long reaction times, and achieve safe and environmentally friendly synthesis processes, broad market prospects, and economic benefits. good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
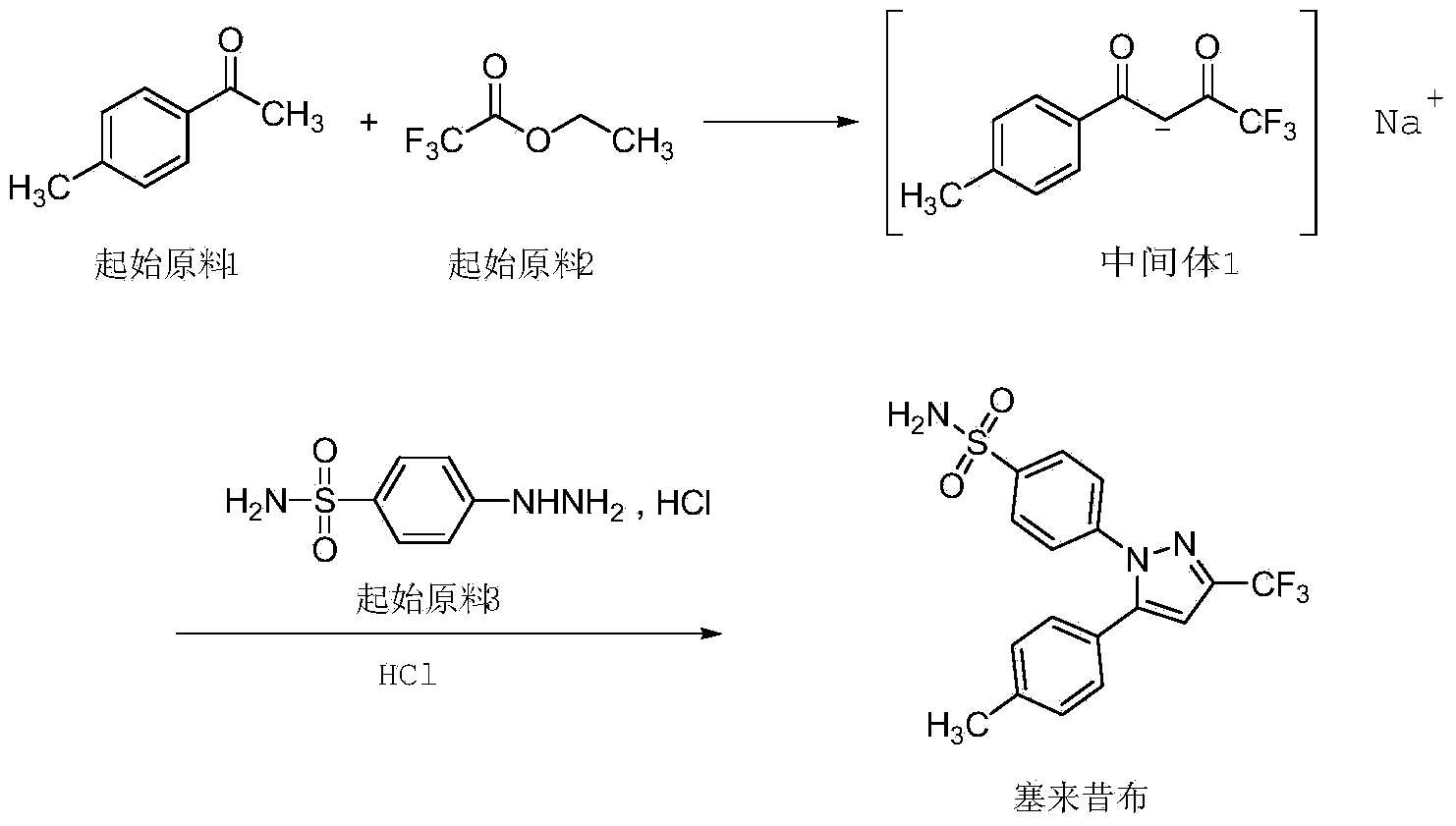
[0028] Add 150ml of ethanol, 40ml (336mmol) of ethyl trifluoroacetate, 18.1g (336mmol) of sodium methoxide into a 1000ml three-neck flask, stir for 5-10 minutes, and then add 30ml (224mmol) of p-methylacetophenone. Raise the temperature to 50°C and react for 1 hour, then p-methylacetophenone was completely reacted by TLC spotting, cooled to room temperature, added 450ml of petroleum ether for crystallization, and obtained 54.0g of a white solid with a purity of 99.1% and a yield of 95.6%.
[0029] In a 1000ml three-necked flask, add 80ml of ethyl acetate and 210ml of isopropanol, 33g (131mmol) of intermediate 1, add 15.8ml of concentrated hydrochloric acid (concentration 37.5%) and stir, then add 29.3g (131mmol) of hydrazinobenzenesulfonamide salt acid salt, heated to 70°C for 1 hour reaction, and then HPLC detected that intermediate 1 was completely reacted, cooled to room temperature, added 500ml of purified water for crystallization, and obtained 44.9g of crude celecoxib wit...
Embodiment 2
[0032] Add 150ml tetrahydrofuran, 40ml (336mmol) ethyl trifluoroacetate, 18.1g (336mmol) sodium methoxide into a 1000ml three-neck flask, stir for 5-10 minutes, then add 30ml (224mmol) p-methylacetophenone. Raise the temperature to 50°C and react for 1 hour, then p-methylacetophenone was completely reacted by TLC spotting, cooled to room temperature, added 480ml of petroleum ether for crystallization, and obtained 46.7g of a white solid with a purity of 99.3% and a yield of 93.5%.
[0033] In a 1000ml three-necked flask, add 80ml of ethyl acetate and 200ml of isopropanol, 33g (131mmol) of intermediate 1, add 16.0ml of concentrated hydrochloric acid (concentration 37.5%) and stir, then add 29.3g (131mmol) of hydrazinobenzenesulfonamide salt After heating up to 60°C to react for 1 hour, the reaction of intermediate 1 was detected by HPLC. After cooling down to room temperature, 530ml of purified water was added for crystallization to obtain 53.1g of crude celecoxib with a yield o...
Embodiment 3
[0036] Add 150ml isopropanol, 40ml (336mmol) ethyl trifluoroacetate, 18.1g (336mmol) sodium methoxide into a 1000ml three-neck flask, stir for 5-10 minutes, then add 30ml (224mmol) p-methylacetophenone. Raise the temperature to 40°C and react for 1 hour, then p-methylacetophenone was completely reacted by TLC spotting, cooled to room temperature, added 390ml of petroleum ether for crystallization, and obtained 53.5g of a white solid with a purity of 99.5% and a yield of 94.8%.
[0037] In a 1000ml three-necked flask, add 80ml of ethyl acetate and 220ml of isopropanol, 33g (131mmol) of intermediate 1, add 15.5ml of concentrated hydrochloric acid (concentration 37.5%) and stir, then add 29.3g (131mmol) of hydrazinobenzenesulfonamide salt acid salt, heated to 60°C for 1 hour reaction, and then HPLC detected that intermediate 1 was completely reacted, cooled to room temperature, added 520ml of purified water for crystallization, and obtained 45.5g of crude celecoxib with a yield of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com