Method for synthesizing loxoprofen sodium
A technology of loxoprofen sodium and its synthetic method, which is applied in the field of medicine and chemical industry, and can solve the problems of low product purity, high price of triethyl orthoformate, complex raw materials, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
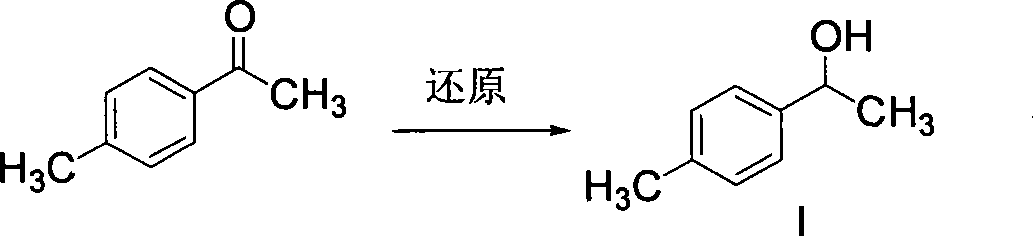
[0101] Example 1 Preparation of 1-(4-methylphenyl)-1-ethanol (compound I)
[0102]6.7g (0.05mol) of 4-methylacetophenone, 30ml of methanol, stir evenly, cool down in an ice-water bath, add 2.28g (0.06mol) of sodium borohydride, react at 30°C for 3 hours, add 20ml of water, stir evenly, water The layer was extracted with dichloromethane, and the layers were separated. The organic phase was washed with water, dried over anhydrous sodium sulfate, filtered, and the filtrate was evaporated to remove the solvent under reduced pressure to obtain 6.56 g of 1-(4-methylphenyl)-1-ethanol (Compound I), with a yield of 96.4%, detected by HPLC The content is more than 99%.
Embodiment 2
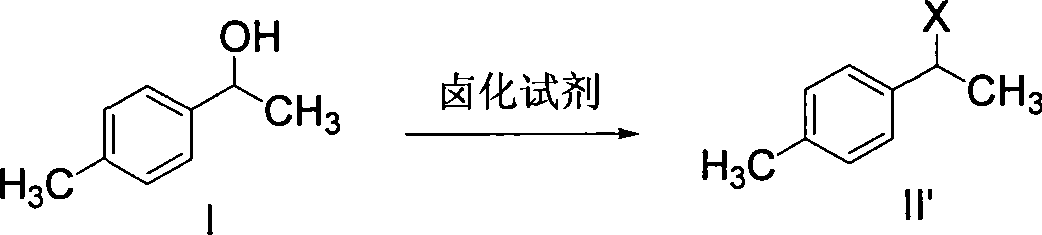
[0103] The preparation of embodiment 2 (1-(4-methylphenyl)-1-ethanol) sulfonate (compound II)
[0104] 6.8g (0.05mol) of compound I, 9.1g (0.09mol) of triethylamine, 30ml of dichloromethane, stirred, and cooled in an ice-water bath. 10.3 g (0.09 mol) of methanesulfonyl chloride was slowly added dropwise at below 15°C. After the dropwise addition was continued and stirred for 3 hours, the heating was stopped, and the layers were separated. The organic phase was washed with saturated sodium bicarbonate until pH = 7, dried over anhydrous sodium sulfate, filtered, and the solvent was evaporated under reduced pressure to obtain 9.81 g of compound II. The yield is 91.7%, and the content detected by HPLC is 95%.
Embodiment 3
[0105] Example 3 Preparation of 1-(4-methylphenyl)-1-chloroethane (compound II')
[0106] Compound I61g (0.448mol), carbon tetrachloride 200ml, thionyl chloride 80g (0.67mol) was added dropwise at room temperature. After dropping, the mixture was heated to reflux for 3 hours. Cool down to room temperature, add an appropriate amount of water to wash the organic phase, and then wash the organic phase with saturated sodium bicarbonate until the pH of the aqueous layer = 7, and add anhydrous sodium sulfate to dry. Filtration, carbon tetrachloride was distilled off under reduced pressure to obtain 65 g of compound II', the yield was 93.5%, and the content detected by HPLC was 97%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com