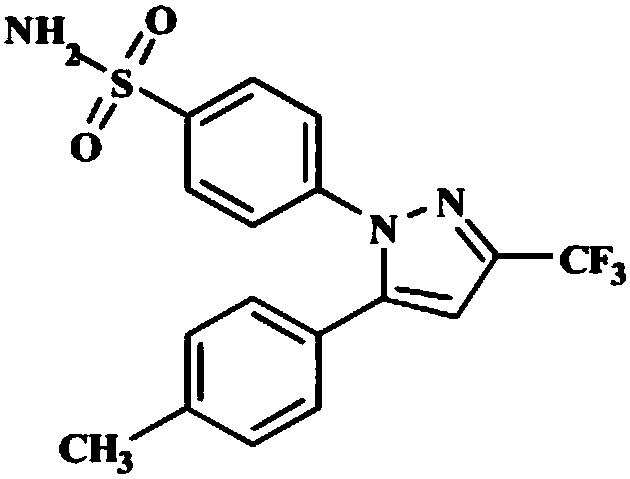
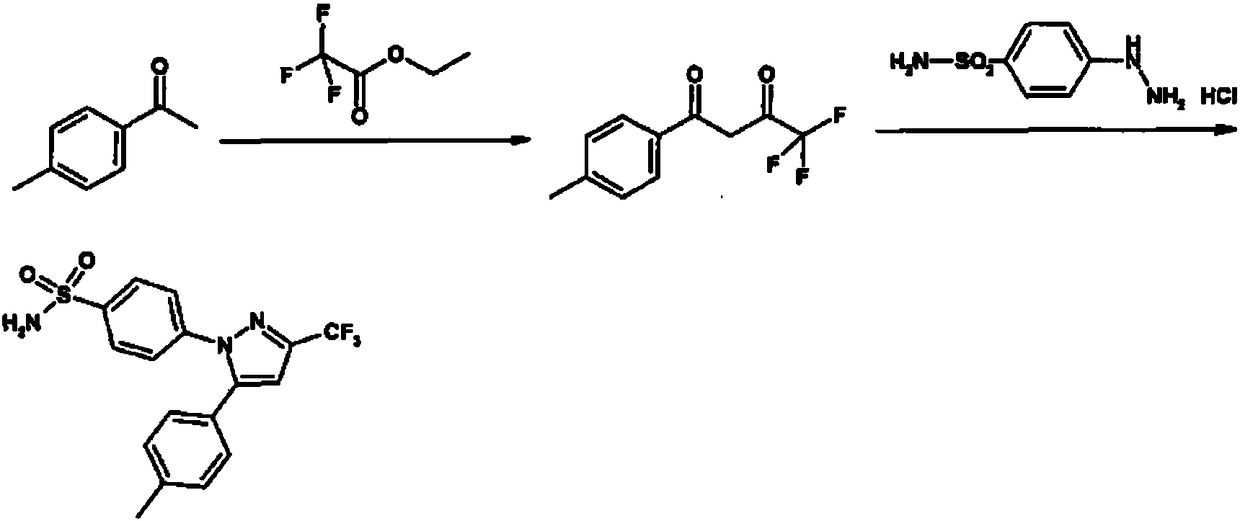
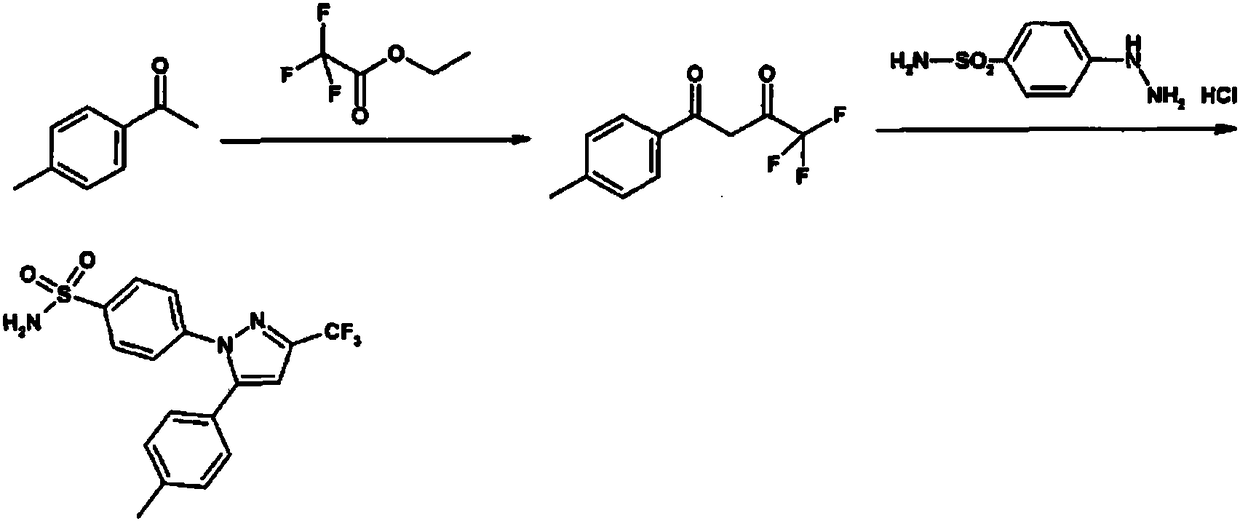
Method for preparing celecoxib by using one-pot method
A technology of celecoxib and p-methylacetophenone, which is applied in the field of medicine, can solve the problems of the price of the recrystallization mixed solution, the unqualified solvent residue of the product, and the difficulty in recycling, so as to reduce the amount of solvent used and the discharge of sewage. amount, reducing the effect of purification and treatment processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 1) Preparation of Intermediate I
[0033] Add 34.6ml (291mmol) ethyl trifluoroacetate and 26.7ml (400mmol) ethylenediamine into a 1000ml three-necked flask, stir for 5-10 minutes, then add 30ml (224mmol) p-methylacetophenone, heat up to 60°C for reaction After 2.5 hours, the reaction of p-methylacetophenone was detected by TLC plate detection, and the intermediate DO was obtained.
[0034] 2) Preparation of celecoxib crude product
[0035] Add 500ml of methanol to the reaction solution in the previous step, add 50.1g (224mmol) of p-hydrazinobenzenesulfonamide hydrochloride, add concentrated hydrochloric acid (concentration: 37.5%) to adjust the pH value to 3.4, and heat the feed solution to 65°C for 3.5 Hours; after the reaction is complete, add 530ml of purified water, cool down to 20°C, keep warm for 1.5 hours to crystallize, and filter with suction. Obtain 77.65g of celecoxib crude product, the purity is 99.5%, and the yield is 90.9%.
[0036] 3) Preparation of Ce...
Embodiment 2
[0040] 1) Preparation of Intermediate I
[0041] Add 35.6ml (300mmol) ethyl trifluoroacetate and 27ml (405mmol) ethylenediamine into a 1000ml three-necked flask, stir for 5-10 minutes, then add 30ml (224mmol) p-methylacetophenone, and heat up to 65°C for reaction 2 Hours, then TLC spot plate detection p-methylacetophenone reaction is complete, obtains intermediate DO.
[0042] 2) Preparation of celecoxib crude product
[0043] Add 450ml of methanol to the reaction solution in the previous step, add 50.0g (223mmol) of p-hydrazinobenzenesulfonamide hydrochloride, add concentrated hydrochloric acid (concentration: 37.5%) to adjust the pH value to 3.3, and heat the feed solution to 60°C for reaction 4 After 1 hour, the reaction was completed, 500ml of purified water was added, the temperature was lowered to 20° C., crystallization was carried out after 1.5 hours of heat preservation, and suction filtration was performed. Obtain 76.8g of celecoxib crude product, the purity is 99....
Embodiment 3
[0048] 1) Preparation of Intermediate I
[0049] Add 340ml (286mmol) ethyl trifluoroacetate and 26.7ml (400mmol) ethylenediamine into a 1000ml three-necked flask, stir for 5-10 minutes, then add 30ml (224mmol) p-methylacetophenone, heat up to 60°C and react for 2.5 Hours, then TLC spot plate detection p-methylacetophenone reaction is complete, obtains intermediate DO.
[0050] 2) Preparation of celecoxib crude product
[0051] Add 500ml of isopropanol to the reaction solution in the previous step, add 49g (21.9mmol) p-hydrazinobenzenesulfonamide hydrochloride, add concentrated sulfuric acid (98%) to adjust the pH value to 3.4, and heat the feed solution to 65°C for 3.5 After 1 hour, the reaction was completed, 550ml of purified water was added, the temperature was lowered to 20°C, the temperature was maintained for 1.5 hours to crystallize, and suction filtration was performed. Obtained celecoxib crude product 75.5g, the purity is 99.5%, the yield is 88.4%.
[0052] 3) Prepar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com