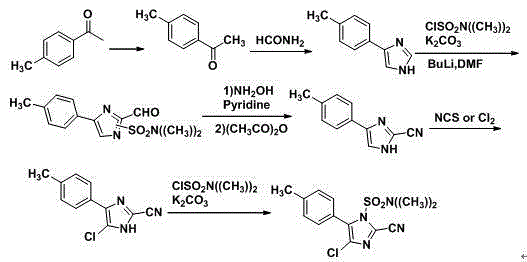
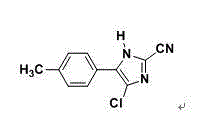
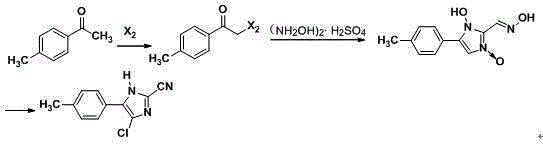Synthetic method for 2-cyano-4-chloro-5-(4-methylphenyl)imidazole
A technology of methyl phenyl and synthetic method, which is applied in the field of synthesis of 2-cyano-4-chloro-5-(4-methylphenyl) imidazole, which can solve the difficulty of industrial production and the safety hazards of industrial production High, difficult operation and other problems, to achieve the effect of high product yield, short reaction time, easy process operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] (1) In the light reactor, add 300L cyclohexane, 135Kg p-methyl acetophenone and 50L hydrogen peroxide, turn on the light, add 170Kg bromine dropwise, the reaction temperature is 20°C, add dropwise for 1 hour, at 20°C Keep it warm for 3 hours, cool and crystallize in an ice bath, and filter with suction to obtain compound Ⅰ;
[0035] (2) In reactor A, add 500L dimethyl sulfoxide and 215Kg compound I, and stir and react at 120°C for 4 hours. After the reaction is completed, cool and crystallize in an ice bath, and obtain compound II by suction filtration;
[0036] (3) In reactor B, add 800L of methanol, 135Kg of glyoxal, 325Kg of hydroxylamine sulfate and 129Kg of compound II, heat up to reflux and keep it warm for 6 hours, the raw materials are completely reacted, crystallized by ice bath cooling, washed with water, and suction filtered to obtain Compound III;
[0037] (4) In reactor C, add 600Kg N,N-dimethylformamide and 188Kg compound III, cool in an ice bath, add 136...
Embodiment 2
[0039] (1) In the light reactor, add 500L cyclohexane, 155Kg p-methyl acetophenone and 70L hydrogen peroxide, turn on the light, add 190Kg bromine dropwise, the reaction temperature is 30°C, add dropwise for 1 hour, at 30°C Keep it warm for 3 hours, cool and crystallize in an ice bath, and filter with suction to obtain compound Ⅰ;
[0040] (2) In reactor A, add 500L dimethyl sulfoxide and 200Kg compound I, stir and react at 120°C for 4 hours, after the reaction is completed, cool and crystallize in an ice bath, and obtain compound II by suction filtration;
[0041] (3) In reactor B, add 800L of methanol, 145Kg of glyoxal, 365Kg of hydroxylamine sulfate and 140Kg of compound II, heat up to reflux and keep it warm for 6 hours, the raw materials are completely reacted, crystallized by ice bath cooling, washed with water, and suction filtered to obtain Compound III;
[0042] (4) In reactor C, add 700Kg N,N-dimethylformamide and 200Kg compound III, cool in an ice bath, add 156Kg t...
Embodiment 3
[0044] (1) In the light reactor, add 300L cyclohexane, 155Kg p-methyl acetophenone and 75L hydrogen peroxide, turn on the light, add 190Kg bromine dropwise, the reaction temperature is 20°C, add dropwise for 1 hour, at 20°C Keep it warm for 3 hours, cool and crystallize in an ice bath, and filter with suction to obtain compound Ⅰ;
[0045] (2) In reactor A, add 500L dimethyl sulfoxide and 228Kg compound I, and stir and react at 110°C for 4 hours. After the reaction is completed, cool and crystallize in an ice bath, and obtain compound II by suction filtration;
[0046] (3) In reactor B, add 800L of methanol, 115Kg of glyoxal, 305Kg of hydroxylamine sulfate and 109Kg of compound II, heat up to reflux and keep it warm for 6 hours, the raw materials are completely reacted, cooled in an ice bath to crystallize, washed with water, and suction filtered to obtain Compound III;
[0047] (4) In reactor C, add 600Kg N,N-dimethylformamide and 168Kg compound III, cool in an ice bath, add...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com