Method for preparing p-methylacetophenone
A technology of p-methyl acetophenone and methyl acetophenone, which is applied in the field of preparation of p-methyl acetophenone, can solve the problems that the catalyst cannot be recycled, the economic benefit is poor, and the equipment is corroded after treatment, etc., and it is easy to scale The effect of modernization and continuous production, simple structure and stable operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
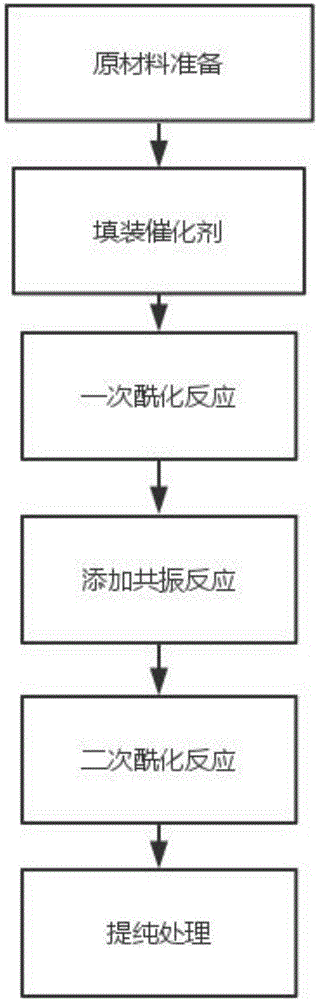
[0020] Such as figure 1 Shown, a kind of preparation method of p-methylacetophenone of the present embodiment comprises following processing steps:
[0021] (1) raw material preparation: the production raw material of this methyl acetophenone comprises following components (parts by weight): 3 parts of toluene, 50 parts of acylating reagents, 100 parts of organic solvents, 5 parts of catalysts, 10 parts of aluminum trichloride, Wherein the acylating reagent comprises acetyl chloride, acetic anhydride and ethyl acetate, wherein the organic solvent is composed of benzene, acetic acid, sulfolane, chlorobenzene and nitrobenzene;
[0022] (2) Filling the catalyst: the catalyst is evenly filled into the fixed bed reactor, and the fixed bed is preheated. The preheating temperature is 120°C, and the preheating time is 10 minutes;
[0023] (3) Primary acylation reaction: send the toluene and acylating reagent in step (1) into the fixed bed reactor in proportion, and pressurize and hea...
Embodiment 2
[0027] Embodiment 2: all the other are identical with embodiment 1, difference is that in described step (1), production raw material comprises following component (weight part): toluene 4 parts, acylating reagent 60 parts, organic solvent 110 parts, catalyst 8 12 parts, 12 parts of aluminum trichloride, the preheating duration in the step (2) is 15min, and the preheating temperature is 1300° C., the pressurized heating pressure in the step (3) is 10 MPa, and the heating temperature is 180° C. The reaction duration is 25min, the reaction duration in the step (4) is 15min, the pressurized pressure in the step (5) is 20MPa, the reaction duration is 40min, and the heating temperature is 280°C, and the rectification in the step (6) The temperature was 170°C.
Embodiment 3
[0028] Embodiment 3: all the other are identical with embodiment 1, difference is that in described step (1), production raw material comprises following component (weight part): 5 parts of toluene, 60 parts of acylating reagents, 120 parts of organic solvents, catalyzer 10 parts part, 15 parts of aluminum trichloride, the preheating duration in the step (2) is 15min, and the preheating temperature is 150°C, the pressurized heating pressure in the step (3) is 15MPa, and the heating temperature is 200°C, The reaction duration is 30min, the reaction duration in the step (4) is 20min, the pressurized pressure in the step (5) is 25MPa, the reaction duration is 50min, and the heating temperature is 300°C, and the rectification in the step (6) The temperature was 180°C.
[0029] After the above process steps, the p-methylacetophenone sample is taken out to obtain the following data:
[0030]
[0031]
[0032] From the above data, it can be seen that the purity of p-methylacet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com